A B S T R A C T
The plants of Celtis L. genus have been traditionally used to cure aches, sore throats, fevers, cancer, sexually transmitted diseases, sexual weakness, diarrhea, stomach problems, amenorrhea, menstrual disorders, kidney stones, and pain. The review aims to give a comprehensive account of the current state of ethnopharmacology, phytochemistry, and biological activities of the Celtis genus, as well as to describe the potential area of future avenues. Information on the Celtis genus was obtained from internet sources such as Google Scholar, Web of Science, PubMed, Science- Direct, and so on by using appropriate keywords, including ethnobotanical, pharmacological, pharmaceutical, bioactivity, phytochemistry, and botanical features of the Celtis genus. This re- view identified 14 species in the genus Celtis that have a phytopharmacological investigation, including C.africana Burm. f., C. australis L., C. occidentalis L., C. sinensis Pers., C. philippensis Blanco., C. tetrandra Roxb., C. tessmannii Rendle., C. jessoensis Koidz., C. adolfi-friderici Engl.,
C. iguanaea (Jacq.) Sarg., C. laevigata Wild., C. pallida Torr., C. zenkeri Engl., and C. tournefortii Lam. This genus contains many classified phytoconstituents, such as terpenoids, organic acids, flavonoids, and volatile compounds. Their extracts and pure substances have been shown to have
the same anticancer, antibacterial, anti-inflammatory, antioxidant, hepatoprotective, car-
dioprotective, urease-inhibiting, and antidiarrheal properties as their traditional uses. In terms of current information on ethnopharmacology, phytochemicals, and pharmacological uses, the data acquired in this review could be beneficial and needed for future research. Some phytocon- stituents (for instance, kaempferol, myricetin, quercetin, and eugenol) and extracts (for example, leaves, seeds, and ripe fruits extracts of C. australis) showed tremendous results in preliminary testing with promising antimicrobial, anticancer, and urease inhibitory effects. Further research and clinical investigations are needed to develop them as lead compounds and neutraceuticals, which may provide an advance over traditional medicinal systems.
Abbreviations
2D-NMR Two-dimensional Nuclear Magnetic Resonance A2780 Human Ovarian Cancer
A549 Adeno-Carcinomic Human Alveolar Basal Epithelial Cells ACF Aberrant Crypt Foci
AGS Human Gastric Adenocarcinoma Cells Bcl2 B-Cell Leukemia 2
CAT Catalase
C-NMR Carbon-13 Nuclear Magnetic Resonance
COX-2 Cyclooxygenase-2
CYP-1A1 Cytochrome P450 Family 1 Subfamily A Member 1 DAD Diode-Array Detection
DPPH 2,2-Diphenyl-1-Picrylhydrazyl
ESI-MS Electrospray Ionization Mass Spectroscopy
ERK1/2 Extracellular Signal-Regulated Kinase ½
EI-MS Electron Ionization Mass Spectroscopy FID Flame Ionization Detector
FT-IR Fourier Transform Infrared Spectroscopy FRAP Ferric Reducing Ability of Plasma
GC-MS Gas Chromatography Mass Spectroscopy GSH Glutathione
HCT-116 Human Colon Cancer Cell line
H-NMR Proton Nuclear Magnetic Resonance
HPLC High-Performance Liquid Chromatography
HR-FAB-MS High-Resolution Fast Atom Bombardment Mass Spectroscopy HRESIMS High-Resolution Electrospray Ionization Mass Spectrometry HREIMS High-Resolution Electron Ionization Mass Spectrometry
HMG-CoA Reductase 3-Hydroxy-3-Methyl-Glutaryl-Coenzyme A Reductase
iNOS Inducible Nitric Oxide Synthase IR Infrared Spectroscopy
JNK Jun N-Terminal Kinases
KAS Beta-Ketoacyl-[acyl Carrier Protein]-Synthase LC-MS Liquid Chromatography Mass Spectroscopy LDL Low-Density Lipoprotein
MIC Minimum Inhibitory Concentration MBC Minimum Bactericidal Concentration mir-26b MicroRNA 26b
mir-146a MicroRNA 146a
MRSA Methicillin-Resistant Staphylococcus aureus
MS Mass Spectroscopy mRNA Messenger RNA
NMDAR N-Methyl-D-Aspartate-Receptor PC-3 Human Prostate Cancer Cell line QS Quorum Sensing
RSH Reactive Thiol Group,
SFE-CO2 Supercritical Fluid Extraction of CO2
SOD Superoxide Dismutase
TBARS Thiobarbituric Acid Reactive Substances TOF/MS Time of Flight Mass Spectroscopy
TRAIL Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand, TNF-α Tumor Necrosis Factor-Alpha
UHPLC Ultra High-Pressure Liquid Chromatography
UV Ultraviolet Spectroscopy
QqQ-MS Triple Quadrupole Mass Spectroscopy BHT Butylated Hydroxytoluene
BHA Butylated Hydroxyanisole
MAPK Mitogen-Activated Protein Kinase
SHP2 Src Homology Region 2 (SH2)-Containing Protein Tyrosine Phosphatase 2 STAT Signal Transducers and Activators of Transcription
Scientific names
B. cereus Bacillus cereus
B. megaterium Bacillus megaterium
B. subtilis Bacillus subtilis
C. albicans Candida albicans
C. freundii Citrobacter freundii
C. neoformans Cryptococcus neoformans
C. parapsilosis Candida parapsilosis
C. tropicalis Candida tropicalis
E. aerogenes Enterobacter aerogenes
E. coli Escherichia coli
K. pneumonia Klebsiella pneumonia
L. ivanovii Listeria ivanovii
L. monocytogenes Listeria monocytogenes
M. avium Mycobacterium avium
M. tuberculosis Mycobacterium tuberculosis
P. aeruginosa Pseudomonas aeruginosa
P. falciparum Plasmodium falciparum
P. mirabilis Proteus mirabilis
P. vulgaris Proteus vulgaris
R. mucilaginosa Rhodotorulamucilaginosa
S. aureus Staphylococcus aureus
P. aeruginosa Pseudomonas aeruginosa
Introduction
Scientists have explored natural sources for discovering novel therapeutic compounds throughout the ages [1–3]. This effort has resulted in the discovery of several therapeutic plants that can potentially cure various diseases [4–6]. Interestingly, almost 80 % of the world’s population relies heavily on natural approaches to health care needs [7–9]. These medicinal plants’ ability to promote re-
covery is due to their varied chemical compounds, which have abundant biological impacts on living beings [10,11]. In particular, these biologically active phytomolecules are the source of many pharmacological medicines [12]. For instance, medicinal plants feature antimalarial molecules like quinine, cardioactive drugs like digoxin, narcotic pain relievers like morphine, and anti-neoplastic therapies like vincristine and vinblastine [13]. Therefore, potent medicinal plant genus may play a vital role in discovering new lead medicinal molecules.
The Celtis genus is one of the potential sources of medicinal compounds that exhibit prosperous ethnopharmacological properties. Almost every portion of these plants (leaves, barks, roots, saps, etc.) historically utilized in traditional treatments for a wide array of
diseases such as diabetics, venereal, gastrointestinal, amenorrhea, pain, headache, and fever [14–26]. A wide range of biochemical
activities have been revealed by preliminary biological and therapeutic assessments of extracts and secondary metabolites of Celtis
species. These encompass anti-cancer, anti-inflammatory, antimicrobial, analgesic, antifungal, antidiabetic, and antioxidant features [25,27–38].
Identified chemicals from Celtis plants show potential in the fight against antimicrobial resistance (AMR), while AMR is an urgent
problem that led to almost 3.57 million deaths worldwide in 2019 [39]. For the managing such AMR threats, the antimicrobial activity of the medicinal plants poses a new hope [40]. Moreover, the antibacterial efficacy of the Celtis plant’s molecules, including eugenol, palmitic acid, and stearic acid has been noted against resistant strains [41,42]. These phytoconstituents could be used as a starting point to find novel antibiotic compounds that can reduce AMR cases.
However, the therapeutic details of Celtis’s compounds is still limited, especially in regard to their efficacy, mode of action,
therapeutic index, and probable toxicity. A thorough analysis of the Celtis genus is required to clarify its present status and inform future investigation scope to the researcher, because most of the findings made until now are in the preliminary stage. While one review has concentrated on a single Celtis species, Celtis australis [43], many other species of the Celtis genus have not been rigorously reviewed. This comprehensive review of the Celtis genus is required to fill this knowledge gap.
Celtis is the genus of hackberries or nettle trees belonging to the Cannabaceae family, is mainly distributed in Africa, Asia, northern Australia, and South and North America [44,45]. Formerly, Celtis plants were allocated as either Ulmaceae or a new family, Celti- daceae. However, Celtis is now classified under the Cannabaceae family [46]. According to the Plant List 2022, 349 scientific names of the genus Celtis are documented, including 69 accepted names, 222 synonym species, and 55 unaccessible data (www.theplantlist.org). This unique genus can be separated from other genera of its family, especially by leaf characteristics: deciduous, alternate, and distichous with three veins rather than one vein. Flowers are small, greenish, and either unisexual or bisexual. Fruits are fleshy and one-seeded [47].
From this comprehensive review, considering the botanical, pharmacological, biological, and phytochemistry aspects of species from the genus Celtis, only 14 species have been evaluated for the extensive analyses as per our knowledge, which include C. africana Burm. f., C. australis L., (synonym: Celtis australis var. eriocarpa Decne.),C. occidentalis L., C. sinensis Pers.,C. philippensis Blanco.,
C. tetrandra Roxb.,C. tessmannii Rendle.,C. jessoensis Koidz., (Synonym: C. choseniana Nakai), C. adolfi-friderici Engl.,C. iguanaea (Jacq.) Sarg. (synonym: C. ehrenbergiana (Klotzsch) Liebm.),C. laevigata Wild., C. pallida Torr., C. zenkeriEngl., andC. tournefortii Lam. (syn- onym: C. aetnensis (Tornab.) Strobl). Among them, C. africana Burm. f., C. australis L., and C. sinensis Pers. were the most often evaluated species across a broad range of ailments. This review aims to gather the present state knowledge from the ethno- pharmacological to the phytopharmacological value of the genus Celtis for future studies. The existing knowledge of phytochemical components with their characterization data and medicinal uses of this genus is reviewed to accelerate the discovery of new lead compounds.
Methods
The relevant data of the genus Celtis was collected via electronic resources such as Google Scholar, PubMed, Web of Science, and ScienceDirect using search terms “ethnobotanical use of Celtis”, “pharmacological use of Celtis”, “pharmaceutical use of Celtis”, “bioactivity of Celtis”, “phytochemistry of Celtis”, and “botanical characteristics of Celtis”. This review included the relevant websites, journal articles, Ph.D. thesis, and books.
From 1881 to 2023, a total of 2514 articles were collected by searching keywords rigorously. Where, only 1479 abstracts were matched with this study’s title and aims. Relevant websites, journal articles, books, and Ph.D. thesis were collected, while 202 pertinent sources were short-listed (Fig. 1). Duplicates, lack of full text, abstract not available in English, withdrawn or retracted articles, lack of ethnopharmacology and phytochemical investigation were eliminated (n 899) (Fig. 1). The details of this review methodology based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) were sketched in Fig. 1.
=
All chemical synonyms were taken from the PubChem database, while synonymous scientific names were taken from the Plant List 2022 website (www.theplantlist.org). Chemical structures of the phytoconstituents were drawn with ChemDraw 16.0 (PerkinElmer Informatics, in Waltham, MA).
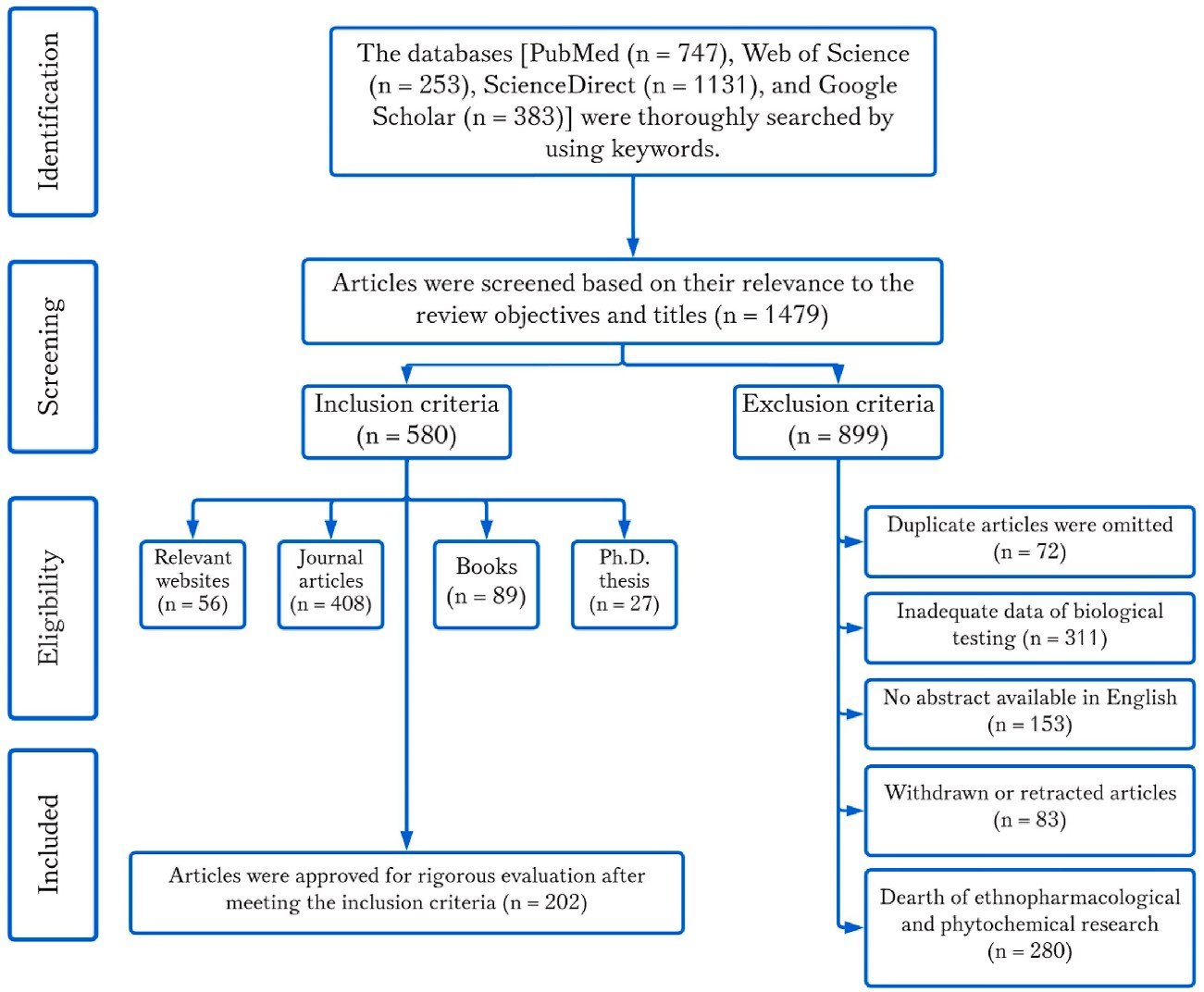
Fig. 1. The article selection procedure following the preferred reporting items for systematic review and meta-analysis (PRISMA) protocols.
Botany and distribution of Celtis genus.
| Scientific name | Distribution | Leaves | Fruits | Flowers | References |
| C. adolphi- | Togo, Benin, Democratic Republic | Alternate, simple, broadly | Fleshy, sub-globose | The flowers are small with a | [49] |
friderici
of Congo, Uganda, Guinea, Ivory Coast, Ghana
elliptic, mesophyll, entire, glabrous
white corolla.
C. africana From West Africa to Sudan,
Arabia, Angola, and the Cape Province of South Africa.
C. australis From West Asia to the
Mediterranean, including Morocco, Spain, Syria, the Caucasus, and Central and Northern Europe.
Simple alternate, egg- shaped, soft hairy, asymmetrical and has three veins raised from the base.
Simple, cauline, alternate, stipulate, and hairy stipules and petiolate.
Yellowish-colored fruits are found from October to February.
Harvested in the autumn and with a single seed
Unisexual, greenish, small, and raised in springs.
From March to April, green, small, unisexual flowers bloom.
[52–54]
C. choseniana North Korea, and South Korea. Pale green, deciduous,
narrow or wide ovate, papery, glabrous upper, and glaucous lower leaves.
Orange-yellow, ellipsoidal to globose, and solitary fruit
Flowers placed in tightly packed cymes
[55]
C. iguanaea From New Mexico east to Virginia,
Illinois south to Florida, and New Mexico west to Virginia.
Native to South America, Central America, and North America
The tops of the leaves are pale greenish-yellow, and the bottoms are pale green.
Oval to broadly elliptic, wide, acute or attenuate at the apex, obtuse to subcordate at the base.
Ovid shaped, orange or brownish-red colored long fruit comes from September to October.
Flowers bloom in mid-May and grow in separate or small clusters.
Greenish-yellow, bisexual, cylindrical ovary, hairy, staminate flowers.
[47,
C. occidentalis In North America, east of
Mississippi, Ontario, and eastern Canada; the Southeastern US; the Southern Appalachian States; and Northwest Italy.
The leaves have three principal veins. oblong or lanceolate in shape.
Reveal in September to October. Purple or brownish, fleshy, thin skin, one seed, globular fruit.
In bloom in April and May [47,56]
C. pallida From the south to the middle of
America, Arizona, Florida, New Mexico, and Texas.
C. philippensis Madagascar, India, Myanmar,
Southeast China, Taiwan, Thailand, Malaysia, Northeast and West Australia, and the Solomon Islands.
Ovate to ovate-oblong shape, rounded apex, rough surfaces.
Elliptical to lanceolate, ovate-elliptical shape
It may be yellow, orange, or red.
The color ranges from orange to red, and the shape ranges from globose to ellipsoid, with an obtusely rounded base.
It blooms from March to May.
Cluster cyme has five bisexual flowers and five or more male flowers.
[59]
C. sinensis China, Taiwan, Korea, and Japan Ovate or ovate-elliptic,
hair scatters from major veins.
Fasciculate in the leaf axils and at the stem bases. Style branches are linear and undivided, and bloom in March or April.
Stone white flowers bloom in September or October.
C. tessmanni Native to Gabon, Cameroon,
Congo, Central Republic of Africa.
Elliptic-shaped leaves Fruits may be orange or
black
Hermaphrodites stay at the apex of cymes crowded with male flowers.
[45]
C. tournefortii Ukraine, Croatia, Greece, Cyprus,
northwestern Iran, northern Iraq, Turkey, and the Caucasus region, Azerbaijan.
Oval to narrowly oval, acute to sub acuminate leaves
Matured fruits are yellow to orange in color.
C. zenkeri From Ivory Coast to Angola,
Uganda, Tanzania
Oblong-elliptic to ovate, shortly acuminate, 3- nerved from the base
Sub-globose or ovoid, red, pubescent or subglabrous.
Lower with clustered male
flowers, often with 1–2 female or hermaphrodite flowers at the top.
[64]
Botany
The Celtis genus is a member of the Plantae kingdom, Viridiplantae subkingdom, Streptophyta infrakingdom, Embry- ophytasuperdivision, Tracheophyta division, Spermatophytina subdivision, Magnoliopsida class, Rosanae superorder, Rosales order, and Cannabaceae family [48].
Table 2
The traditional uses of Celtisgenus.
preparation
| Species | Part used | Method of | Medicinal uses | Region | References |
| C. adolphi- | Barks | Decoction | General malaise, severe cough, fever and headache, and | N/A | [65] |
friderici
as an emetic
Barks Pulp Relieve costal and side pains of chest Democratic republic
of Congo
[65]
Barks, fruits, and leaves
N/A Tuberculosis, severe cough, headache, fever, and sore eyes
Cameroon [49]
Fruits N/A Tuberculosis Democratic republic of Congo
[65]
Leaves Decoction Sore eyes N/A [65]
Roots N/A Sexual impotence Ghana [66]
C. africana Bark and roots Dry powder Cancer South Africa [16]
Infused in water or milk
| Ground Bark | N/A | General pain, headache, and fever | Nigeria | [15] |
| Leaves | Direct Consumption | Trypanosomiasis edema (Cattle) | Kenya | [14] |
| Leaves | Pounded leaves | Indigestion (Cattle) | Mali | [14] |
| Leaves | N/A | Pleurisy | Lesotho | [15] |
| Leaves | N/A | Indigestion, edema | South Africa | [67] |
| N/A | N/A | Rheumatism, pains, syphilis, cancer | South Africa | [17,18] |
| Bark | Decoction | Astringent for peptic ulcers, dysentery, and diarrhea | India | [23] |
| Barks | Paste | Bones, pimples, contusions, sprains and joint pains | India | [19] |
| Fruits | N/A | Amenorrhea, colic, heavy menstrual and intermenstrual | India | [20,21] |
C. australis
bleeding
Leaves and fruits Decoction Peptic ulcers, dysentery, diarrhea, heavy menstrual and
intermenstrual bleeding, and amenorrhea
India [19]
Roots Boiling Colic and other stomach troubles India [23,24] Stems & Leaves Crushing Leprosy India [22]
N/A N/A Gastrointestinal problems Morocco [25]
C. choseniana Leaves N/A Inflammation exposure Korean [68]
C. ehrenbergiana Leaves Infusion Indigestion N/A [69]
C. eriocarpa Bark Grounded powder Sprain, pimples and India [70]
Joint pain
Barks Powdered bark Tumor, scabies and skin problems Kashmir [71] Seeds Dry seeds Dysentery Kashmir [71]
Fruits N/A Amenorrhea and colic India [72]
Leaves Decoction Amenorrhea Pakistan [73]
C. iguanaea Bark N/A Fever Brazil [74] Fruits Decoction Dysentery and intestinal catarrh Brazil [75]
Fruits Sap Eye diseases N/A [76] Leaves Infusion Used as a vaginal douche to treat leucorrhea Brazil [75] Leaves and fruits Aqueous infusion Kidney pain Ecuador [77]
Leaves and flowers
Infusion Diabetes mellitus Mexico [78]
Leaves and roots Decoction Urinary tract infections Brazil [79]
N/A Use as tea Body aches, rheumatism, chest pain, asthma, cramps,
poor digestion, diuretics
Brazil [80]
C. laevigata Barks Boiling liquor Sore throats America [81] Barks Powdered shells Venereal diseases America [81]
C. occidentalis Barks Decoction Menses and sore throat America [81]
Barks Decoction &
powdered shells
Venereal Diseases America [81]
Wood
Extracts
Jaundice Canada [26]
C. pallida Stems and Leaves
Dry Powder Stomach aches, diarrhea, inflammation, wounds,
cholera, pain, coughing, and skin infections
Mexico [36]
C. philippensis Leaves Saps Parasitic infections N/A [82] Roots N/A Ulcer Tanzania [83]
C. sinensis Barks Decoction Lumbago, menstruation irregularity, gastric problems,
abdominal pain
Korea [84]
Leaves Decoction, paste Lacquer sore, urticaria, eczema Korea [84]
Root barks N/A Dyspepsia, poor appetite, shortness of breath, and swollen feet
China [21]
C. tessmannii Bark Decoction Diabetes and hypertension problem Cameroon [85] Stem bark Decoction Diabetes mellitus Gabon [86]
N/A Malaria, gangrene, sexual weakness, insomnia, and nervosity
N/A Tachycardia, anemia, respiratory inflammation, analgesics, fever, and diarrhea
Cameroon [85]
Cameroon [85] (continued on next page)
Table 2 (continued )
preparation
| Species | Part used | Method of | Medicinal uses | Region | References |
| C. tetrandra | Excluding root, | N/A | Used as a contraceptive for semen | N/A | [87] |
plants coagulation properties
Seeds Juice Indigestion Nepal [88]
Shoots and leaves
N/A Loss of appetite N/A [82]
Roots N/A Laxative N/A [82]
Tender leaves Vegetables Reducing postpartum pains India [89]
C. tournefortii Seeds N/A Kidney sand Turkey [33]
Leaves N/A Stomach pain, cessation of bleeding, inducing sedation, and digestion
Turkey [33]
Fruits N/A Diarrhea, dysentery, and ulcer Turkey [33]
C. zenkeri Stem-bark Decoction Cough, arthritis, fever Nigeria [90–92] Steam-bark Powdered Analgesic Nigeria [90–92]
Wood Macerated Cuts on the skin Nigeria [92]
Celtis plants have axillary spines and can be evergreen or deciduous, polygamo-monoecious, or monoecious. The leaves are alternate and have a whole or toothed margin and three veins from the base. Inflorescences might be clustered into cymelets, racemes, or paniculates. Flowers are small, and either unisexual or bisexual. The inflorescences are made up of branched racemes or panicles.
Flowers are 4–5 merous, with basally slightly connate tepals in male flowers, caducous, and sessile ovaries. The fruit is fleshy with a
wild, foliaceous, and variably folded seed leaf that ranges in size from 3 to 25 mm [45]. Characteristics of flowers, fruits, leaves and distribution of the Celtis plants are given in Table 1.
Ethnopharmacology
Celtis species are being used to treat a variety of diseases almost all around the world. Approximately all parts of Celtis plants are traditionally used to treat various ailments. These parts are processed as decoctions, powdered shells, extracts, and boiling liquor for medicinal purposes (Table 2).
Almost all investigated Celtis species are used to treat pains, sore throats, fevers, diarrhea, and stomach problems (Table 2). The stems and leaves of C. australis and C. pallida, as well as the leaves of C. philippensis, are applied in various forms to treat skin-related problems [36,82,93]. Furthermore, venereal diseases such as sexually transmitted diseases and sexual weakness are treated with
C. africana and the barks of C. occidentalis in the forms of decoction and powdered shells [81,94]. Decoctions of the barks of
C. occidentalis and fruits of C. australis are used to treat menstrual problems such as menses, amenorrhea, heavy menstrual, and intermenstrual bleeding [20,21,81]. The dried barks and roots of C. africana are applied in powder form and infused in water or milk to treat cancer [16]. In Cameroon, the barks, fruits, and leaves of C. adolphi-friderici are used to treat tuberculosis, sore eyes, fever, cough,
and headaches [49]. Another species, C. ehrenbergiana leaves’ infusion is used to treat indigestion [69]. Additionally, the leaves and
fruits of C. iguanaea and the seeds of C. tournefortii are also used to make aqueous infusions for treating kidney problems such as pain and sand [33,77]. These traditional uses suggested that Celtis plants may contain compounds with a wide range of biological activities such as analgesic, antimicrobial, anti-inflammatory, anticancer, antioxidant (protective), anti-fibrinolytic, and anti-diarrhea.
Phytochemistry
Among the numerous species of Celtis plant, only a few have been studied for their phytoconstituents. Although phytochemicals can be found in various parts of the plant, they are mainly found in three principal segments: leaves, stems, and roots. The percentage composition of every plant varies based on preparation techniques, ecological factors, and variety [95]. Flavonoids, tannins, alkaloids, and phenolic constituents are the most common molecules found in phytochemical investigations [96]. Other compounds such as terpenoids, fatty acids, esters, aldehydes, alcohols, and their glycosides are also reported to be present in these plants (Table 3).
Diverse phytochemicals are found in the aerial parts, fruits, leaves, stems, barks, roots, seeds, and twigs of these plants. A study in Saudi Arabia identified amide, fatty acids, terpenoids, sterol [102], and flavonoids [123]in the aerial parts of C. africana, while al- cohols, aldehydes, ketones, and esters were found in the leaves, fruits, and stems in a South African study in addition to fatty acids, terpenoids, and sterol [27].
C. australis leaves, fruits, barks, and stems contain phytochemical elements that are substantially similar to those found in
C. africana, such as phenolic acids, fatty acids, flavonoids, terpenoids, and sterols [31,32,111,122,127]. The ripe fruits and seeds of
C. australis contain various types of esters, and fatty acids [31,32], while the fruits of C. tournefortii contain phenolic acid, benzoic acid, fatty acids, esters, tannins, terpenoids, and flavonoids [33,113,114]. C. pallida possess alcohol, fatty acids, esters, terpenoids, sugars [36], phenolic acids, and flavonoids [117].
In a Hungarian study of dried extract of C. occidentalis, amides were identified in the twigs [100], while an Egyptian study of ethanol extract identified several flavonoid compounds [122]. Dichloromethane-ethanol extracts of C. iguanaea leaves contain
Table 3
Phytochemistry of Celtis genus.
| Compound | Chemical class | Compound | Species | Organs | Extract | Structure elucidation | Collection | Rf no. |
| Number | site |
Amides
- Ceramide Celtisamide A C. tessmannii Stem barks Methanol extract NMR, UV, IR, MS, GC-MS
- Ceramide Celtisamide B C. tessmannii Stem barks Methanol extract NMR, UV, IR, MS, GC-MS
Cameroon [97]
Cameroon [97]
- Fatty acid derivatives Oleamide C. sinensis Leaves and stems
SFE-CO2 GC-MS China [98]
C. zenkeri Leaves GC-MS Nigeria [99]
- Hydroxycinnamic acid derivatives
2-trans-3-(4-hydroxyphenyl)- N-[2-(4- hydroxyphenyl)-2- oxoethyl] prop-2-enamide
C. occidentalis Twigs Methanol extract UHPLC-Orbitrap-MS, H-NMR, C-NMR,
Hungary [100]
- Hydroxycinnamic acid derivatives
- Hydroxycinnamic acid derivatives
- Hydroxycinnamic acid derivatives
cis-N-coumaroyltyramine C. sinensis Twigs Methanol extract H-NMR, C-NMR, FT-
IR, UV
trans-N-caffeoyltyramine C. africana Aerial parts Ethanol-water extract H-NMR, C-NMR,
EIMS, HREIMS
C. occidentalis Twigs Methanol extract UHPLC-Orbitrap-MS, H-NMR, C-NMR,
C. sinensis Twigs Methanol extract H-NMR, C-NMR, FT- IR, UV
C. tessmannii Stem barks Methanol extract NMR, UV, IR, MS, GC-MS
trans-N-coumaroyloctopamine C. occidentalis Twigs Methanol extract UHPLC-Orbitrap-MS,
H-NMR, C-NMR,
C. tessmannii Stem barks Methanol extract NMR, UV, IR, MS, GC-MS
Korea [101]
Saudi Arabia [102]
Hungary [100]
Korea [101]
Cameroon [97]
Hungary [100]
Cameroon [97]
- Hydroxycinnamic acid derivatives
trans-N-coumaroyltyramine C. adolphi- friderici
Roots Acetone extract Cameroon [103]
C. africana Aerial parts Ethanol-water extract H-NMR, C-NMR,
EIMS, HREIMS
C. occidentalis Twigs Methanol extract UHPLC-Orbitrap-MS, H-NMR, C-NMR,
C. sinensis Twigs Methanol extract EI-MS, H-NMR, C- NMR
C. tessmannii Stem barks Methanol extract NMR, UV, IR, MS, GC-MS
C. zenkeri Stem barks Methanol extract HREIMS, C-NMR, H- NMR
Saudi Arabia [102]
Hungary [100]
Korea [104]
Cameroon [97]
[90]
- Hydroxycinnamic acid derivatives
trans-N-feruloyloctopamine C. adolphi- friderici
Roots Acetone extract Cameroon [103]
C. occidentalis Twigs Methanol extract UHPLC-Orbitrap-MS, H-NMR, C-NMR,
C. tessmannii Roots Methanol extract NMR, UV, IR, MS, GC-MS
Hungary [100]
Cameroon [97]
Hydroxycinnamic acid derivatives
trans-N-feruloyltyramine C. adolphi- friderici
Roots Acetone extract Cameroon [103]
C. africana Aerial parts Ethanol-water extract H-NMR, C-NMR,
M.A. Samadd et al.
Heliyon 10 (2024) e29707
8
EIMS, HREIMS
C. occidentalis Twigs Methanol extract UHPLC-Orbitrap-MS, H-NMR, C-NMR,
Saudi Arabia [102]
Hungary [100] (continued on next page)
Table 3 (continued )
M.A. Samadd et al.
Heliyon 10 (2024) e29707
9
| Compound Number | Chemical class | Compound | Species | Organs | Extract | Structure elucidation | Collection | Rf no. |
| 11. | Iso-benzo-furanone | Zenkeramide | C. tessmannii
C. zenkeri |
Roots
Stem-barks |
Methanol extract
Methanol |
NMR, UV, IR, MS,
H-NMR, C-NMR, |
Cameroon
Nigeria |
[97]
[90] |
| Esters
12. |
propanamide
Anthraquinone ester |
6-hydroxy-5,7,8-trimethoxy-9,10-dioxo-9,10- | C. australis | Stem barks | Ethanol extract | HREIMS
H-NMR, C-NMR, IR, |
India | [105] |
| 13. | Carboxylic ester | dihydroanthracen-2-yl acetate 2-Propenoic acid, butyl ester | C. sinensis | & Fruits Leaves and | SFE-CO2 | MS
GC-MS |
China | [98] |
| 14. Carboxylic ester | Benzyl benzoate | C. africana | stems
Stems |
Dichloromethane: methanol extract | 2D-GC-TOF/MS | South Africa | [27] | |
| 15. Carboxylic ester | Malic acid, 4-ethyl ester | C. pallida | Aerial parts | Ethanol extract | GC-MS | Mexico | [36] | |
| 16. Carboxylic ester | Methyl salicylate | C. sinensis | Leaves and | SFE-CO2 | GC-MS | China | [98] | |
| 17. Ester | Sulfurous acid, dibutyl ester | C. africana | stems
Leaves |
Hexane extract | 2D-GC-TOF/MS | South Africa | [27] | |
| 18. Fatty acid ester | 1,2-Benzenedicarboxylic acid, butyl oxtyl ester | C. sinensis | Leaves and | SFE-CO2 | GC-MS | China | [98] | |
| 19. Fatty acid ester | 2-Methylstearoate | C. australis | stems
Ripe Fruits |
Ethanol extract | FT-IR, GC-MS | India | [31] | |
| 20. Fatty acid ester | Acetic acid n-octadcyl ester | C. sinensis | Leaves and | SFE-CO2 | GC-MS | China | [98] | |
| 21. | Fatty acid ester | Arachidic acid methyl ester | C. tourneforti | stems
Leaves and |
Hexane extract | GC-MS | Iraq | [106] |
| 22. | Fatty acid ester | Capric acid methyl ester | C. tourneforti | fruits
Leaves and |
Hexane extract | GC-MS | Iraq | [106] |
| 23. | Fatty acid ester | Dibutyl phthalate | C. sinensis | fruits Leaves and | SFE-CO2 | GC-MS | China | [98] |
| 24. | Fatty acid ester | Diethyl phthalate | C. sinensis | stems
Leaves and |
SFE-CO2 | GC-MS | China | [98] |
| 25. Fatty acid ester | Ethyl linolenate | C. pallida | stems
Aerial parts |
Ethanol extract | GC-MS | Mexico | [36] | |
| 26. Fatty acid ester | Ethyl palmitate | C. africana | Leaves | Hexane extract | 2D-GC-TOF/MS | South Africa | [27] | |
| 27. Fatty acid ester | Glycerol 1-stearate | C. adolphi- | Roots | Acetone extract | FAB-MS, EI-MS, H- | Cameroon | [103] | |
| 28. | Fatty acid ester | Hexadecanoic, 2-hydroxyethyl ester | friderici
C. pallida C. sinensis |
Aerial parts Leaves and | Ethanol extract SFE-CO2 | NMR GC-MS GC-MS | Mexico China | [36]
[98] |
| 29. Fatty acid ester | Hexacosyl heptafluorobutyrate | C. zenkeri | stems
Leaves |
Methanol | GC-MS | Nigeria | [107] | |
| 30. Fatty acid ester | Lignoceric acid methyl ester | C. tourneforti | Leaves and | Hexane extract | GC-MS | Iraq | [106] | |
| 31. | Fatty acid ester | Linoleic acid-, 2-hydroxy-1-(hydroxymethyl) | C. africana | fruits
Fruits |
Dichloromethane: methanol extract | 2D-GC-TOF/MS | South Africa | [27] |
| ethyl ester | ||||||||
| 32. Fatty acid ester | Linolenic acid, methyl ester | C. africana | Fruits | Ethyl acetate Extract | 2D-GC-TOF/MS | South Africa | [27] | |
| 33. Fatty acid ester | Methyl 13-methyltetradecanoate | C. australis | Ripe fruits | Ethanol extract | FT-IR, GC-MS | India | [31] | |
| 34. Fatty acid ester | Methyl 14-acetyl hydroxy palmitate | C. australis | Ripe fruits | Ethanol extract | FT-IR, GC-MS | India | [31] | |
| 35. Fatty acid ester | Methyl 1-dotriacontanoate | C. australis | Ripe Fruits | Ethanol extract | FT-IR, GC-MS | India | [31] | |
| 36. Fatty acid ester | Methyl 1-tetradecanoate | C. australis | Ripe fruits | Ethanol extract | FT-IR, GC-MS | India | [31] | |
| 37. Fatty acid ester | Methyl 2,4-dimethyl heneicosanoate | C. australis | Ripe fruits | Ethanol extract | FT-IR, GC-MS | India | [31] | |
| 38. Fatty acid ester | Methyl dotriacentanoate | C. australis | Ripe fruits | Ethanol extract | FT-IR, GC-MS | India | [31] | |
| 39. Fatty acid ester | Methyl linoleate | C. australis | Ripe Fruits | Ethanol extract | FT-IR, GC-MS | India | [31] | |
| 40. Fatty acid ester | Methyl oleate | C. australis | Ripe fruits | Ethanol extract | FT-IR, GC-MS | India | [31] | |
| C. zenkeri | Leaves | GC-MS | Nigeria | [31] | ||||
site
GC-MS
(continued on next page)
Table 3 (continued )
M.A. Samadd et al.
Heliyon 10 (2024) e29707
10
| Compound Number | Chemical class | Compound | Species | Organs | Extract | Structure elucidation | Collection | Rf no. |
| 41. | Fatty acid ester | Methyl Palmitate | C. australis | Ripe fruits | Ethanol extract | FT-IR, GC-MS | India | [31] |
| C. iguanaea | Leaves | Dichloromethane and ethanol | GC-MS | Brazil | [108] | |||
| 42. Fatty acid ester | Methyl pentachloro stearate | C. australis | Ripe fruits | Ethanol extract | FT-IR, GC-MS | India | [31] | |
| 43. Fatty acid ester | Methyl stearate | C. australis | Ripe fruits | Ethanol extract | FT-IR, GC-MS | India | [31] | |
| C. iguanaea | Leaves | Dichloromethane and ethanol | GC-MS | Brazil | [108] | |||
| extract | ||||||||
| 44. Fatty acid ester | Methyl tetradecanoate | C. australis | Ripe fruits | Ethanol extract | FT-IR, GC-MS | India | [31] | |
| 45. Fatty acid ester | Methyl tricosanoate | C. australis | Ripe fruits | Ethanol extract | FT-IR, GC-MS | India | [31] | |
| C. tourneforti | Leaves and | Hexane extract | GC-MS | Iraq | [106] | |||
| fruits | ||||||||
| 46. Fatty acid ester | Monolinolenin | C. africana | Fruits | Dichloromethane: methanol extract | 2D-GC-TOF/MS | South Africa | [27] | |
| 47. Fatty acid ester | Phthalic acid, butyl 2-ethylhexyl ester | C. sinensis | Leaves and | SFE-CO2 | GC-MS | China | [98] | |
| 48. | Fatty acid ester | Phthalic acid, butyl tetradecyl ester | C. sinensis | stems
Leaves and |
SFE-CO2 | GC-MS | China | [98] |
| 49. | Fatty acid ester | Phthalic acid, di-isobutyl ester | C. sinensis | stems
Leaves and |
SFE-CO2 | GC-MS | China | [98] |
| 50. Fatty acid ester | Stigmast-5-en-3-ol oleate | C. ehrenbergiana | stems
Leaves |
Crude methanolic extract | GC-MS | Brazil | [109] | |
| 51. Hydroxycinnamic | Chlorogenic acid | C. australis | Fruits | Methanol extract | HPLC | Iran | [110] | |
| acid ester | C. australis | Leaves | RP-HPLC, UV | Italy | [111] | |||
| C. iguanaea | Leaves | 70 % ethanol | HPLC | Brazil | [112] | |||
| C. tournefortii | Fruits | Methanol extract | HPLC, UV | Turkey | [113] | |||
| C. tournefortii | Fruits & | Methanol–dichloromethane extract | HPLC-TOF/MS | Turkey | [114] | |||
| 52. | Phenolic ester | Protocatechuic acid, ethyl ester | C. tournefortii | Leaves
Fruits, |
Methanol–dichloromethane extract | HPLC-TOF/MS | Turkey | [114] |
| 53. | Triterpene ester | 3β-trans-sinapoyloxylup-20(29)-en-28-ol | C. philippinensis | Leaves &
Twigs |
Methanol extract | FT-IR, HR-FAB-MS, | Indonesia | [115] |
| 54. | Triterpene ester | 3β-trans-feruloyloxy-16β-hydroxylup-20(29)- | C. philippinensis | Twigs | Methanol extract | H-NMR, C-NMR
FT-IR, HR-FAB-MS, |
Indonesia | [115] |
| Flavonoids
55. |
Anthocyanin | ene
Cyanidin-3,5-di-O-glucoside |
C. australis | fruits & | Water and ethanol extract | H-NMR, C-NMR
UHPLC–QqQ-MS/ |
Croatia | [32] |
| 56. | Anthocyanin | Delphinidin-3,5-di-O-glucoside | C. australis | Leaves
fruits & |
Water extract | MS, UV
UHPLC–QqQ-MS/ |
Croatia | [32] |
| 57. | Anthocyanin | Pelargonidin-3,5-di-O-glucoside | C. australis | Leaves
fruits & |
Water and ethanol extracts | MS, UV
UHPLC–QqQ-MS/ |
Croatia | [32] |
| 58. | Flavanol | Afzelechin | C. tetrandra | Leaves
Barks |
Ethyl acetate extract | MS, UV
MS, H-NMR, C-NMR, |
Thailand | [116] |
| 59. | Flavanol | Catechin | C. pallida | Leaves & | Methanol, methanol-water or | HRESIMS
HPLC |
Mexico | [117] |
| Fruits | acetone extract | |||||||
| C. tetrandra | Barks | Ethyl acetate extract | MS, H-NMR, C-NMR, | Thailand | [116] | |||
| HRESIMS | ||||||||
| C. tournefortii | Fruits | Methanol extract | HPLC, UV | Turkey | [113] | |||
| C. tournefortii | Leaves & | Methanol–dichloromethane extract | HPLC-TOF/MS | Turkey | [114] | |||
| Young twigs | ||||||||
site
extract
Young twigs
(continued on next page)
Table 3 (continued )
Compound Number
Chemical class Compound Species Organs
Extract
Structure elucidation Collection
site
Rf no.
- Flavanol Epiafzelechin C. tetrandra Barks Ethyl acetate extract MS, H-NMR, C-NMR,
HRESIMS
- Flavanol Epicatechin C. australis Leaves Ethanol extract UHPLC–QqQ-MS/ MS, UV
Thailand [116]
Croatia [32]
C. pallida Leaves &
Fruits
Methanol, methanol-water or acetone extract
HPLC Mexico [117]
- Flavanol Gallocatechin C. pallida Leaves Methanol, methanol-water or acetone extract
HPLC Mexico [117]
- Flavanol dimer Epiafzelechin-(4α→8)-catechin C. tetrandra Barks Ethyl acetate extract MS, H-NMR, C-NMR,
HRESIMS
- Flavanol dimer Epiafzelechin-(4α→8)-epicatechin C. tetrandra Barks Ethyl acetate extract MS, H-NMR, C-NMR,
HRESIMS
Thailand [116]
Thailand [116]
- Flavanone Naringenin C. tournefortii Fruits Water, ethanol and methanol extract
RP-HPLC-DAD, UV Turkey [33]
C. tournefortii Fruits, Leaves & Young twigs
methanol–dichloromethane extract HPLC-TOF/MS Turkey [114]
- Flavanone glycoside Eriodictyol acetyl-glucoside- pentoside C. eriocarpa leaves Methanol extract UHPLC-DAD, ESI-MS Pakistan [118]
- Flavanone glycoside Hesperidin C. tournefortii Fruits, Leaves & Young twigs
Methanol–dichloromethane extract HPLC-TOF/MS Turkey [114]
- Flavanone glycoside Naringenin glucuronide glucoside C. eriocarpa Leaves methanol extract UHPLC-DAD, ESI-MS Pakistan [118]
- Flavanone glycoside Naringin C. tournefortii Fruits Water, ethanol and methanol extract
RP-HPLC-DAD, UV Turkey [33]
C. tournefortii Fruits, Leaves &Young twigs
- Flavanone glycoside Neohesperidin C. tournefortii Leaves &
Young twigs
Methanol–dichloromethane extract HPLC-TOF/MS Turkey [114]
Methanol–dichloromethane extract HPLC-TOF/MS Turkey [114]
- Flavanonol Taxifolin C. tournefortii Fruits Methanol–dichloromethane extract HPLC-TOF/MS Turkey [114]
- Flavone Acacetin C. eriocarpa Leaves Methanol extract UHPLC-DAD, ESI-MS Pakistan [118]
- Flavone Apigenin C. australis Fruits Ethanol extract EIMS, IR, H-NMR, C- NMR
India [119]
C. australis Fruits Methanol extract HPLC Iran [110]
C. tournefortii Fruits, Leaves & Young twigs
Methanol–dichloromethane extract HPLC-TOF/MS Turkey, Iraq [106,
114]
- Flavone Diosmetin C. tournefortii Young twigs Methanol–dichloromethane extract HPLC-TOF/MS Turkey [114]
- Flavone Hispidulin C. australis Leaves Methanol extract LC-MS Montenegreo [120]
- Flavone Luteolin C. choseniana Leaves Methanol extract HPLC Korea [68]
- Flavone Wogonin C. tournefortii Fruits, Leaves & Young twigs
Methanol–dichloromethane extract HPLC-TOF/MS Turkey [114]
- Flavone glycoside 2″-O-α-L-rhamnopyranosyl-7-O-methylvitexin C. australis Leaves RP-HPLC, UV Italy [111]
- Flavone glycoside 2-O-pentosyl-8-C-hexosyl-apigenin C. iguanaea Leaves Dichloromethane extract ESI-MS Brazil [121]
- Flavone glycoside 2″-O-β-D-galactopyranosyl orientin C. australis Leaves Ethanol extract UV, HRESIMS, 1D-
NMR, 2D-NMR
Egypt [122]
(continued on next page)
M.A. Samadd et al.
Heliyon 10 (2024) e29707
11
Table 3 (continued )
M.A. Samadd et al.
Heliyon 10 (2024) e29707
12
| Compound Number | Chemical class | Compound | Species | Organs | Extract | Structure elucidation | Collection | Rf no. |
| 81. | Flavone glycoside | 2″-O-β-galactopyranosyl vitexin | C. occidentalis
C. australis |
Leaves
Leaves |
Ethanol extract
Ethanol extract |
UV, HRESIMS, 1D-
UV, HRESIMS, 1D- |
Egypt
Egypt |
[122]
[122] |
| C. occidentalis | Leaves | Ethanol extract | NMR, 2D-NMR
UV, HRESIMS, 1D- |
Egypt | [122] | |||
| NMR, 2D-NMR | ||||||||
| 82. Flavone glycoside | 2-α-rhamnopyranosyl vitexin | C. eriocarpa | Leaves | Methanol extract | UHPLC-DAD, ESI-MS | Pakistan | [118] | |
| 83. Flavone glycoside | 4‴-rhamnosyl-2″-O-β-D-galactopyranosyl vitexin | C. australis | Leaves | Ethanol extract | UV, HRESIMS, 1D- | Egypt | [122] | |
| C. occidentalis | Leaves | Ethanol extract | NMR, 2D-NMR
UV, HRESIMS, 1D- |
Egypt | [122] | |||
| 84.
85. |
Flavone glycoside Flavone glycoside | Acacetin 7-O-glucoside Acacetin-8-C-rutinoside | C. australis
C. eriocarpa |
Leaves Leaves | Methanol extract | NMR, 2D-NMR RP-HPLC, UV
UHPLC-DAD, ESI-MS |
Italy Pakistan | [111]
[118] |
| 86. | Flavone glycoside | Apigenin 6-C-glucoside | C. australis | Leaves | RP-HPLC, UV | Italy | [111] | |
| 87. Flavone glycoside | Apigenin 7-O-galloylrhamnoside | C. eriocarpa | Leaves | Methanol extract | UHPLC-DAD, ESI-MS | Pakistan | [118] | |
| 88. Flavone glycoside | Apigenin-6,8-di-C-glucoside | C. eriocarpa | Leaves | Methanol extract | UHPLC-DAD, ESI-MS | Pakistan | [118] | |
| 89. Flavone glycoside | Apigenin-6,8-di-C-rhamnoside | C. eriocarpa | Leaves | Methanol extract | UHPLC-DAD, ESI-MS | Pakistan | [118] | |
| 90. Flavone glycoside | Apigetrin | C. australis | Leaves | Methanol extract | LC-MS | Montenegreo | [120] | |
| C. tournefortii | Leaves & | Methanol–dichloromethane extract | HPLC-TOF/MS | Turkey | [114] | |||
| 91. Flavone glycoside | Baicalein dipentosidehexoside | C. eriocarpa | Young twigs
Leaves |
Methanol extract | UHPLC-DAD, ESI-MS | Pakistan | [118] | |
| 92. Flavone glycoside | Baicalein-8-C-glucoside | C. eriocarpa | Leaves | Methanol extract | UHPLC-DAD, ESI-MS | Pakistan | [118] | |
| 93. Flavone glycoside | Baicalin | C. tournefortii | Leaves & | Methanol–dichloromethane extract | HPLC-TOF/MS | Turkey | [114] | |
| 94. Flavone glycoside | Celtiside A | C. africana | Young twigs
Aerial parts |
Ethanol and water extract | 1D NMR, 2D NMR | Saudi Arabia | [123] | |
| 95. Flavone glycoside | Celtiside B | C. africana | Aerial parts | Ethanol and water extract | 1D NMR, 2D NMR | Saudi Arabia | [123] | |
| 96. Flavone glycoside | Dihydroluteolin-7-O-glucoronide | C. eriocarpa | Leaves | Methanol extract | UHPLC-DAD, ESI-MS | Pakistan | [118] | |
| 97. Flavone glycoside | Diosmin | C. tournefortii | Leaves & | Methanol–dichloromethane extract | HPLC-TOF/MS | Turkey | [114] | |
| 98. | Flavone glycoside | Isoorientin | C. australis | Young twigs
Leaves |
Ethanol extract | UV, HRESIMS, 1D- | Egypt | [122] |
| C. occidentalis | Leaves | Ethanol extract | UV, HRESIMS, 1D- | Egypt | [122] | |||
| NMR, 2D-NMR | ||||||||
| 99. Flavone glycoside | Isoswertiajaponin | C. africana | Aerial parts | Ethanol and water extract | 1D NMR, 2D NMR | Saudi Arabia | [123] | |
| 100. Flavone glycoside | Isoswertisin | C. africana | Aerial parts | Ethanol and water extract | 1D NMR, 2D NMR | Saudi Arabia | [123] | |
| 101. | Flavone glycoside | Isovitexin | C. australis | Leaves | RP-HPLC, UV | Italy | [111] | |
| C. australis | Ethanol extract | UV, HRESIMS, H- | Egypt | [122] | ||||
| NMR, C-NMR | ||||||||
| C. occidentalis | Leaves | Ethanol extract | UV, HRESIMS, 1D- | Egypt | [122] | |||
| C. sinensis | Leaves | Ethanol extract | NMR, 2D-NMR | China | [124] | |||
| 102. Flavone glycoside | Isovitexinhydroxyferuloyl glucoside | C. eriocarpa | Leaves | Methanol extract | UHPLC-DAD, ESI-MS | Pakistan | [118] | |
| 103. Flavone glycoside | Luteolin-4 -O-rhamnosyl (1 → 2) glycoside | C. iguanaea | Leaves | Dichloromethane extract | ESI-MS | Brazil | [121] | |
| 104. Flavone glycoside | Luteolin-6-C-acetyl pentoside | C. eriocarpa | Leaves | Methanol extract | UHPLC-DAD, ESI-MS | Pakistan | [118] | |
| 105. Flavone glycoside | Orientin | C. africana | Aerial parts | Ethanol and water extract | 1D NMR, 2D NMR | Saudi Arabia | [123] | |
| C. australis | Leaves | Ethanol extract | UV, HRESIMS, 1D- | Egypt | [122] | |||
| NMR, 2D-NMR | ||||||||
| C. occidentalis | Leaves | Ethanol extract | UV, HRESIMS, 1D- | Egypt | [122] | |||
| NMR, 2D-NMR | ||||||||
site
NMR, 2D-NMR
NMR, 2D-NMR
(continued on next page)
Table 3 (continued )
Compound Number
Chemical class Compound Species Organs
Extract
Structure elucidation Collection
site
Rf no.
C. iguanaea Leaves Dichloromethane extract ESI-MS Brazil [121]
106. Flavone glycoside Scutellarin C. tournefortii Fruits, Leaves & Young twigs
| 107. Flavone glycoside | Tetrahydroxy isoflavone-O-hexoside | C. iguanaea | Leaves | Dichloromethane extract | ESI-MS | Brazil | [121] |
| 108. Flavone glycoside | Vitexin | C. africana | Aerial parts | Ethanol & water extract | 1D NMR, 2D NMR | Saudi Arabia | [123] |
Methanol–dichloromethane extract HPLC-TOF/MS Turkey [114]
C. australis Leaves RP-HPLC, UV Italy [111]
C. australis Ethanol extract UV, HRESIMS, 1D- NMR, 2D-NMR
| C. eriocarpa | Leaves | Methanol extract | UHPLC-DAD, ESI-MS | Pakistan | [118] |
| C. iguanaea | Leaves | Dichloromethane extract | ESI-MS | Brazil | [121] |
| C. occidentalis | Leaves | Ethanol extract | UV, HRESIMS, 1D- | Egypt | [122] |
| C. africana | Aerial parts | Ethanol and water extract | NMR, 2D-NMR
1D NMR, 2D NMR |
Saudi Arabia | [123] |
| C. eriocarpa | Leaves | Methanol extract | UHPLC-DAD, ESI-MS | Pakistan | [118] |
| C. tournefortii | Leaves & | Methanol–dichloromethane extract | HPLC-TOF/MS | Turkey | [114] |
Egypt [122]
109. Flavone glycoside Vitexin 2″-O-rhamnoside
Young twigs
| 110. | Flavonol | Fisetin |
| 111. | Flavonol | Galangin |
| 112. | Flavonol | Kaempferol |
| 113. | Flavonol | Morin |
| 114. | Flavonol | Myricetin |
| 115. | Flavonol | Quercetin |
C. tournefortii Leaves &
Young twigs
Methanol–dichloromethane extract HPLC-TOF/MS Turkey [114]
C. tournefortii Fruits Water, ethanol and methanol
extract
RP-HPLC-DAD, UV Turkey [33]
C. tournefortii Leaves &
Young twigs
Methanol–dichloromethane extract HPLC-TOF/MS Turkey [114]
C. choseniana Leaves Methanol extract HPLC Korea [68]
C. tournefortii Fruits Water, ethanol and methanol
extract
RP-HPLC-DAD, UV Turkey [33]
C. tournefortii Leaves &
Young twigs
Methanol–dichloromethane extract HPLC-TOF/MS Turkey [114]
C. tournefortii Fruits Methanol extract HPLC, UV Turkey [113]
C. tournefortii Young twigs Methanol–dichloromethane extract HPLC-TOF/MS Turkey [114]
C. australis Fruits Ethanol extract EIMS, IR, H-NMR, C- NMR
India [119]
C. choseniana Leaves Methanol extract HPLC Korea [68]
C. ehrenbergiana Leaves Lyophilized aqueous, and crude
methanolic extract
GC-MS Brazil [109]
C. iguanaea Leaves 70 % Ethanol HPLC Brazil [112]
C. tournefortii Fruits Water, ethanol and methanol
extract
RP-HPLC-DAD, UV Turkey [33]
C. tournefortii Leaves &
Young twigs
Methanol–dichloromethane extract HPLC-TOF/MS Turkey [114]
- Flavonol glycoside Isorhamnetin hexosidepentoside C. eriocarpa Leaves Methanol extract UHPLC-DAD, ESI-MS Pakistan [118]
- Flavonol glycoside Kaempferol 3-O-glucoside C. australis Leaves Methanol extract LC-MS Montenegreo [120]
- Flavonol glycoside Quercetin rhamnosidedipentoside C. eriocarpa Leaves Methanol extract UHPLC-DAD, ESI-MS Pakistan [118]
- Flavonol glycoside Quercetin-3-β-D-glucoside C. tournefortii Fruits, Leaves & Young twigs
Methanol–dichloromethane extract HPLC-TOF/MS Turkey [114]
- Flavonol glycoside Rutin C. australis Fruits Methanol extract HPLC Iran [110]
C. australis Leaves Ethanol extract UV, HRESIMS, 1D- NMR, 2D-NMR
M.A. Samadd et al.
Heliyon 10 (2024) e29707
13
Egypt [122]
(continued on next page)
Table 3 (continued )
site
| Compound Number | Chemical class | Compound | Species | Organs | Extract | Structure elucidation | Collection | Rf no. |
| C. iguanaea | Leaves | 70 % Ethanol extract | HPLC | Brazil | [112] | |||
| C. occidentalis | Leaves | Ethanol extract | UV, HRESIMS, 1D- | Egypt | [122] |
C. tournefortii Fruits Water, ethanol and methanol
extract
NMR, 2D-NMR
RP-HPLC-DAD, UV Turkey [33]
C. tournefortii Fruits Methanol extract HPLC, UV Turkey [113]
C. tournefortii Fruits, Leaves & Young twigs
Methanolic solution with 1 % acetic acid
HPLC Iraq [106]
C. tournefortii Fruits, Leaves & Young twigs
Methanol–dichloromethane extract HPLC-TOF/MS Turkey [114]
C. iguanaea Leaves Dichloromethane extract ESI-MS Brazil [121]
- Flavonolignan Silibinin C. tournefortii Young twigs Methanol–dichloromethane extract HPLC-TOF/MS Turkey [114]
- Isoflavone Biochanin A C. tournefortii Fruits, Leaves & Young twigs
Methanol–dichloromethane extract HPLC-TOF/MS Turkey [114]
C. tournefortii Leaves Methanol extract LC-MS/MS Mardin [125]
- Isoflavone glycoside Genistin C. iguanaea Leaves Dichloromethane extract ESI-MS Brazil [121]
Organic acids
- Aliphatic carboxylic acid
5-hydroxypipecolic acid C. ehrenbergiana Leaves Crude methanolic extract GC-MS Brazil [109]
- Aliphatic carboxylic acid
Azelaic acid C. adolphi-
friderici
Roots Acetone extract ESIHRMS, EI-MS, H- NMR
Cameroon [103]
- Aliphatic carboxylic acid
Fumaric acid C. tournefortii Fruits, Leaves & Young twigs
Methanol–dichloromethane extract HPLC-TOF/MS Turkey [114]
- Aliphatic carboxylic acid
- Aliphatic dicarboxylic acid
Methyl quinic acid C. eriocarpa Leaves Methanol extract UHPLC-DAD, ESI-MS Pakistan [118] Quinic acid C. eriocarpa Leaves Methanol extract UHPLC-DAD, ESI-MS Pakistan [118]
- Aliphatic dicarboxylic acid
Sebacic acid C. adolphi-
friderici
Roots Acetone extract EI-MS, H-NMR Cameroon [103]
- Aliphatic dicarboxylic acid
Shikimic Acid C. tournefortii Leaves Methanol extract LC-MS/MS Mardin [125]
- Aliphatic dicarboxylic acid
Succinic acid C. tessmannii Roots Methanol extract NMR, UV, IR, MS, GC-MS
Cameroon [97]
- Benzoic acid
4-Hydroxybenzoic acid C. tournefortii Fruits, Leaves & Young twigs
Methanol–dichloromethane extract HPLC-TOF/MS Turkey [114]
- Benzoic acid
Hydroxybenzoic acid C. adolphi- friderici
Roots Acetone extract Cameroon [103]
| 135. Dicarboxylic acid | Tartaric acid quinylhydroxybenzoylglucoronide | C. eriocarpa | Leaves | Methanol extract | UHPLC-DAD, ESI-MS | Pakistan | [118] |
| 136. Dihydroxy-benzoic | Gentisic acid | C. laevigata | Leaves | Aqueous extract | UV, | United States | [126] |
Carboxylic acid metabolites
Allantoin C. adolphi-
friderici
Roots Acetone extract Cameroon [103]
acid
M.A. Samadd et al.
Heliyon 10 (2024) e29707
14
C. tournefortii Fruits, Leaves &
Chromatographed
Methanol–dichloromethane extract HPLC-TOF/MS Turkey [114]
(continued on next page)
Table 3 (continued )
site
| Compound Number | Chemical class | Compound | Species | Organs | Extract | Structure elucidation | Collection | Rf no. |
| 137. | Dihydroxy-benzoic | Protocatechuic acid | C. tournefortii | Young twigs | Methanol–dichloromethane extract | HPLC-TOF/MS | Turkey | [114] |
| 138. | acid
Dihydroxy-benzoic |
Vanillic acid | C. australis
C. australis |
Leaves
Leaves |
Methanol extract
Ethanol extracts |
LC-MS
UHPLC–QqQ-MS/ |
Montenegreo
Croatia |
[120]
[32] |
| acid
C. australis |
Leaves | Hydro-methanolic extract | MS, UV
H-NMR, C-NMR, |
Morocco | [127] | |||
| C. adolphi- | Roots | Acetone extract | H-NMR, C-NMR, | Cameroon | [103] | |||
| friderici | ||||||||
| C. tournefortii | Fruits | Water, ethanol and methanol | RP-HPLC-DAD, UV | Turkey | [33] | |||
| extract | ||||||||
| C. tournefortii | Fruits, | Methanol–dichloromethane extract | HPLC-TOF/MS | Turkey | [114] | |||
| Leaves & | ||||||||
| 139. | Fatty acid | 2-hydroxy linoleic acid | C. eriocarpa | Young twigs
Leaves |
Methanol extract | UHPLC-DAD, ESI-MS | Pakistan | [118] |
| 140. | Fatty acid | Behenic acid | C. pallida | Aerial parts | Ethanol extract | GC-MS | Mexico | [36] |
| 141. | Fatty acid | Heptacosanoic acid | C. adolphi- | Roots | Acetone extract | EI-MS, H-NMR | Cameroon | [103] |
| 142. | Fatty acid | Hexacosanoic acid | friderici
C. pallida |
Aerial parts | Ethanol extract | GC-MS | Mexico | [36] |
| 143. | Fatty acid | Hydroxy linolenic acid | C. eriocarpa | Leaves | Methanol extract | UHPLC-DAD, ESI-MS | Pakistan | [118] |
| 144.
145. |
Fatty acid
Fatty acid |
Lacceroic acid
Lauric acid |
C. adolphi- friderici
C. africana |
Roots
Aerial parts |
Acetone extract
Ethanol-water extract |
EIHRMS, EI-MS, H-
H-NMR, C-NMR, |
Cameroon
Saudi Arabia |
[103]
[102] |
| 146. | Fatty acid | Lignoceric acid | C. pallida | Aerial parts | Ethanol extract | EIMS, HREIMS
GC-MS |
Mexico | [36] |
| 147. | Fatty acid | Linoleic acid | C. africana | Leaves, | Hexane extract, Ethyl acetate | 2D-GC-TOF/MS | South Africa | [27] |
| Fruits &
Stems |
extract, Dichloromethane: | |||||||
| C. australis | Seeds | Water and ethanol extracts | UHPLC–QqQ-MS/ | Croatia | [32] | |||
| C. pallida | Aerial parts | Ethanol extract | MS, UV
GC-MS |
Mexico | [36] | |||
| C. tournefortii | Fruits | Water, ethanol and methanol | GC, FID | Turkey | [33] | |||
| 148. | Fatty acid | Linolenic acid | C. africana | Fruits, | extract
Hexane extract, Ethyl acetate |
2D-GC-TOF/MS | South Africa | [27] |
| Leaves & | extract, dichloromethane: methanol | |||||||
NMR
methanol extract
Stems extract
C. ehrenbergiana Leaves Crude methanolic extract GC-MS Brazil [109]
C. pallida Aerial parts Ethanol extract GC-MS Mexico [36]
C. tournefortii Fruits Water, ethanol and methanol
extract
| 149. | Fatty acid | Margaric acid | C. pallida | Aerial parts | Ethanol extract | GC-MS | Mexico | [36] |
| 150. | Fatty acid | Myristic acid | C. eriocarpa | Leaves | Methanol extract | UHPLC-DAD, ESI-MS | Pakistan | [118] |
| 151. | Fatty acid | Nonadecanoic acid | C. eriocarpa | Leaves | Methanol extract | UHPLC-DAD, ESI-MS | Pakistan | [118] |
| 152. | Fatty acid | Octacosanoic acid | C. pallida | Aerial parts | Ethanol extract | GC-MS | Mexico | [36] |
| 153. | Fatty acid | Oleic acid | C. africana | Aerial parts | Ethanol-water extract | H-NMR, C-NMR, | Saudi Arabia | [102] |
| C. australis | Seeds | Water and ethanol extracts | UHPLC–QqQ-MS/ | Croatia | [32] | |||
| C. tournefortii | Fruits | Water, ethanol and methanol | MS, UV
GC, FID |
Turkey | [33] | |||
| extract | ||||||||
GC, FID Turkey [33]
M.A. Samadd et al.
Heliyon 10 (2024) e29707
15
EIMS, HREIMS
(continued on next page)
Table 3 (continued )
Compound Number
Chemical class Compound Species Organs
Extract
Structure elucidation Collection
site
Rf no.
- Fatty acid Palmitic acid C. africana Aerial parts Ethanol-water extract H-NMR, C-NMR, EIMS, HREIMS
Saudi Arabia [102]
C. africana Leaves, Fruits & Stems
Hexane extract, Ethyl acetate extract, Dichloromethane: methanol extract
2D-GC-TOF/MS South Africa [27]
C. australis Seeds Water and ethanol extracts UHPLC–QqQ-MS/
MS, UV
Croatia [32]
C. ehrenbergiana Leaves Crude methanolic extract GC-MS Brazil [109]
C. pallida Aerial parts Ethanol extract GC-MS Mexico [36]
C. sinensis Leave &
Stems
SFE-CO2 GC-MS China [98]
C. tournefortii Fruits Water, ethanol and methanol
extract
- Fatty acid Palmitoleic acid C. tournefortii Fruits Water, ethanol and methanol extract
GC, FID Turkey [33]
GC, FID Turkey [33]
- Fatty acid Stearic acid C. australis Seeds Water and ethanol extracts UHPLC–QqQ-MS/
MS, UV
Croatia [32]
C. pallida Aerial parts Ethanol extract GC-MS Mexico [36]
C. sinensis Leaves and stems
SFE-CO2 GC-MS China [98]
C. tournefortii Fruits Water, ethanol and methanol
extract
GC, FID Turkey [33])
Hydroxycinnamic acid Hydroxycinnamic acid Hydroxycinnamic
Aesculetin C. australis Leaves Methanol extract LC-MS Montenegreo [120] Benzoyl sinapic acid C. eriocarpa Leaves Methanol extract UHPLC-DAD, ESI-MS Pakistan [118]
Caffeic acid C. australis Fruits Methanol extract HPLC Iran [110]
acid
C. laevigata Leaves Aqueous extract UV, Chromatographed
United states [126]
C. pallida Fruits Methanol, methanol-water or
acetone extract
C. tournefortii Fruits Water, ethanol and methanol
extract
HPLC Mexico [117]
RP-HPLC-DAD, UV Turkey [33]
C. tournefortii Fruits Methanol extract HPLC, UV Turkey [113]
C. tournefortii Fruits, Leaves & Young twigs
Methanol–dichloromethane extract HPLC-TOF/MS Turkey [114]
Hydroxycinnamic
Cinnamic acid C. australis Fruits Methanol extract HPLC Iran [110]
acid
C. pallida Fruits Methanol, methanol-water or
acetone extract
C. tournefortii Leaves Methanol solution with 1 % acetic
acid
HPLC Mexico [117]
HPLC Turkey [106]
Hydroxycinnamic acid Hydroxycinnamic
Hydroxy-caffeic acid C. eriocarpa Leaves Methanol extract UHPLC-DAD, ESI-MS Pakistan [118]
p-coumaric acid C. australis Fruits Methanol extract HPLC Iran [110]
acid
C. laevigata Leaves Aqueous extract UV, Chromatographed
United states [126]
C. tournefortii Fruits Methanol extract HPLC, UV Turkey [113]
M.A. Samadd et al.
Heliyon 10 (2024) e29707
16
(continued on next page)
Table 3 (continued )
site
| Compound Number | Chemical class | Compound | Species | Organs | Extract | Structure elucidation | Collection | Rf no. |
| C. tournefortii | Leaves & | Methanol–dichloromethane extract | HPLC-TOF/MS | Turkey | [114] | |||
| 163.
164. |
Hydroxycinnamic
acid Hydroxycinnamic |
p-Coumaric acid-O-glucoside
Phenyl caffeic acid |
C. eriocarpa
C. eriocarpa C. eriocarpa |
Young twigs
Leaves Leaves Leaves |
Methanol extract
Methanol extract Methanol extract |
UHPLC-DAD, ESI-MS
UHPLC-DAD, ESI-MS UHPLC-DAD, ESI-MS |
Pakistan
Pakistan Pakistan |
[118]
[118] [118] |
| 165. | acid Hydroxycinnamic | Sinapic acid | C. tournefortii | Leaves & | Methanol–dichloromethane extract | HPLC-TOF/MS | Turkey | [114] |
| 166. | acid
Hydroxycinnamic acid glycoside |
Rosmarinic acid | C. tournefortii | Young twigs
Fruits |
Water, ethanol and methanol | RP-HPLC-DAD, UV | Turkey | [33] |
| 167. Phenolic acid | Dehydro-acacetin dihydroxybenzoic acid | C. eriocarpa | Leaves | Methanol extract | UHPLC-DAD, ESI-MS | Pakistan | [118] | |
| 168. Phenolic acid | Quinic acid phenol | C. eriocarpa | Leaves | Methanol extract | UHPLC-DAD, ESI-MS | Pakistan | [118] | |
| 169. Phenolic acid | Quinoyl galloyl tartaric acid | C. eriocarpa | Leaves | Methanol extract | UHPLC-DAD, ESI-MS | Pakistan | [118] | |
| 170. Phenolic acid | Quinyl malic acid | C. eriocarpa | Leaves | Methanol extract | UHPLC-DAD, ESI-MS | Pakistan | [118] | |
| 171. Phenolic acid | Quinylvanilyl malic acid | C. eriocarpa | Leaves | Methanol extract | UHPLC-DAD, ESI-MS | Pakistan | [118] | |
| 172. Phenolic acid | Syringic acid quinylrhamnoside | C. eriocarpa | Leaves | Methanol extract | UHPLC-DAD, ESI-MS | Pakistan | [118] | |
| 173. Trihydroxy- benzoic | Gallic acid | C. australis | Fruits & | Water extract | UHPLC–QqQ-MS/ | Croatia | [32] | |
| acid
C. australis |
leaves
Fruits |
Methanol extract | MS, UV
HPLC |
Iran | [110] | |||
| C. ehrenbergiana | Lyophilized aqueous and crude | GC-MS | Brazil | [109] | ||||
| methanolic extract | ||||||||
| C. iguanaea | Leaves | 70 % Ethanol | HPLC | Brazil | [112] | |||
| C. pallida | Leaves & | Methanol, methanol-water or | HPLC | Mexico | [117] | |||
extract
Terpenoids
| 174. | Bacterial pentacyclic | 3β-hydroxy-35-(cyclohexyl-50-propan-70-one)- | C. australis | Barks | Ethanol extract | IR, 2D NMR, ESI-MS, | India | [128] |
| 175. | triterpenoid
Carotenoid |
33-ethyl-34-methylbactereohopane
Lutein |
C. australis | Fruits | Water and ethanol extracts | n LCMS QTOF
UHPLC–QqQ-MS/ |
Croatia | [32] |
| 176. | Carotenoid | Zeaxanthin | C. australis | Fruits | Water and ethanol extracts | MS, UV
UHPLC–QqQ-MS/ |
Croatia | [32] |
| 177. | Carotenoid | β-carotene | C. australis | Fruits | Water and ethanol extracts | MS, UV
UHPLC–QqQ-MS/ |
Croatia | [32] |
| 178. | Diterpene | Phytol | C. africana | Leaves | Ethyl acetate extract, | MS, UV
2D-GC-TOF/MS |
South Africa | [27] |
| C. iguanaea | Leaves | Dichloromethane: methanol extract
Dichloromethane and ethanol |
GC-MS | Brazil | [108] | |||
| C. pallida | Aerial parts | extract
Ethanol extract |
GC-MS | Mexico | [36] | |||
| C. zenkeri | Leaves, | GC-MS | Nigeria | [99] | ||||
| 179. | Diterpene | Retinol | C. tournefortii | Stem-bark
Fruits |
Water, ethanol and methanol | RP-HPLC-DAD, UV | Turkey | [33] |
| 180. | Tocopherol | ç-Tocopherol | C. africana | Leaves | extract
Hexane extract |
2D-GC-TOF/MS | South Africa | [27] |
| 181. | Tocopherol | α-Tocopherol | C. africana | Stems &
Leaves |
Ethyl acetate extract, | 2D-GC-TOF/MS | South Africa | [27] |
Fruits
C. tournefortii Fruits, Leaves, & Young twigs
acetone extract
Methanol–dichloromethane extract HPLC-TOF/MS Turkey [114]
M.A. Samadd et al.
Heliyon 10 (2024) e29707
17
Dichloromethane: methanol extract
(continued on next page)
Table 3 (continued )
site
| Compound Number | Chemical class | Compound | Species | Organs | Extract | Structure elucidation | Collection | Rf no. |
| C. australis | Fruits | Water and ethanol extracts | UHPLC–QqQ-MS/ | Croatia | [32] |
MS, UV
C. ehrenbergiana Leaves Crude methanolic extract GC /MS Brazil [109]
C. tournefortii Fruits Water, ethanol and methanol
extract
RP-HPLC-DAD, UV Turkey [33]
C. pallida Aerial parts Ethanol extract GC-MS Mexico [36]
- Tocopherol γ-tocopherol C. australis Fruits Water and ethanol extracts UHPLC–QqQ-MS/ MS, UV
- Tocopherol δ-tocopherol C. australis Fruits Water and ethanol extracts UHPLC–QqQ-MS/ MS, UV
Croatia [32]
Croatia [32]
C. tournefortii Fruits Water, ethanol and methanol
extract
RP-HPLC-DAD, UV Turkey [33]
- Triterpenoid (3β)-3-hydroxy-30-propylhopan-31-one
C. australis Stem barks
& Fruits
Ethanol extract H-NMR, C-NMR, IR, MS
India [105]
- Triterpenoid (3β)-oleanan-3-ol C. australis Stem barks
& Fruits
- Triterpenoid (9β,31R)-9,25-cyclo-30-propylhopan-31-ol C. australis Stem barks
& Fruits
Ethanol extract H-NMR, C-NMR, IR, MS
Ethanol extract H-NMR, C-NMR, IR, MS
India [105]
India [105]
- Triterpenoid 20-epibryonolic acid C. philippinensis Twigs Methanol extract FT-IR, HR-FAB-MS,
H-NMR, C-NMR
- Triterpenoid 3β-O-(E)-coumaroylbetulin C. philippinensis Twigs Methanol extract FT-IR, HR-FAB-MS,
H-NMR, C-NMR
- Triterpenoid 3β-O-(E)-feruloylbetulin C. philippinensis Twigs Methanol extract FT-IR, HR-FAB-MS,
H-NMR, C-NMR
- Triterpenoid Betulin C. philippinensis Twigs Methanol extract FT-IR, HR-FAB-MS, H-NMR, C-NMR
- Triterpenoid Betulinic acid C. tessmannii Stem barks Methanol extract NMR, UV, IR, MS, GC-MS
Indonesia [115]
Indonesia [115]
Indonesia [115]
Indonesia [115]
Cameroon [97]
- Triterpenoid Epifriedelanol C. iguanaea Barks Ethanol extract H-NMR, C-NMR Brazil [129]
C. sinensis Twigs Methanol extract H-NMR, C-NMR, FT- IR, UV,
Korea [101]
- Triterpenoid Friedelin C. adolphi- friderici
Roots Acetone extract Cameroon [103]
C. africana Stems Ethyl acetate extract,
Dichloromethane: methanol extract
2D-GC-TOF/MS South Africa [27]
C. iguanaea Barks Ethanol extract H-NMR, C-NMR Brazil [129]
C. tessmannii Stem barks Methanol extract NMR, UV, IR, MS, GC-MS
Cameroon [97]
- Triterpenoid Friedelinol C. africana Stems Hexane extract, Ethyl acetate extract, Dichloromethane: methanol extract
2D-GC-TOF/MS South Africa [27]
- Triterpenoid Germanicol C. sinensis Twigs Methanol extract H-NMR, C-NMR, FT- IR, UV,
- Triterpenoid Lupeol C. africana Aerial parts Ethanol-water extract H-NMR, C-NMR, EIMS, HREIMS
Korea [101]
Saudi Arabia [102]
C. pallida Aerial parts Ethanol extract GC-MS Mexico [36]
- Triterpenoid Oleanolic acid C. africana Aerial parts Ethanol-water extract H-NMR, C-NMR,
M.A. Samadd et al.
Heliyon 10 (2024) e29707
18
EIMS, HREIMS
Saudi Arabia [102] (continued on next page)
Table 3 (continued )
M.A. Samadd et al.
Heliyon 10 (2024) e29707
19
| Compound Number | Chemical class | Compound | Species | Organs | Extract | Structure elucidation | Collection | Rf no. |
| 198. | Triterpenoid | Platanic acid | C. tessmannii
C. tessmannii |
Stem barks
Stem barks |
Methanol extract
Methanol extract |
NMR, UV, IR, MS,
NMR, UV, IR, MS, |
Cameroon
Cameroon |
[97]
[97] |
| 199. | Triterpenoid | Squalene | C. africana | Leaves | Dichloromethane: methanol extract | GC-MS
2D-GC-TOF/MS |
South Africa | [27] |
| 200. | Triterpenoid | Ursolic acid | C. ehrenbergiana
C. pallida C. pallida |
Aerial parts Aerial parts | Crude methanolic extract Ethanol extract
Ethanol extract |
GC-MS
GC-MS GC-MS |
Brazil
Mexico Mexico |
[109]
[36] [36] |
| C. philippinensis | Twigs | Methanol extract | FT-IR, HR-FAB-MS, | Indonesia | [115] | |||
| C. tessmannii | Stem barks | Methanol extract | NMR, UV, IR, MS, | Cameroon | [97] | |||
| 201. | Triterpenoid | (3β,9β)-9,25-cycloolean-12-en-3-yl β-D- | C. australis | Stem barks | Ethanol extract | GC-MS
H-NMR, C-NMR, IR, |
India | [105] |
| glycoside | glucofuranoside | & Fruits | MS | |||||
| Miscellaneous compounds
202. Acid anhydrate |
2-Dodecen-1-yl (—) succinic anhydride | C. sinensis | Leaves and | SFE-CO2 | GC-MS | China | [98] | |
| 203. Acid anhydrate | Hydroxy-benzoyl p-coumaric acid anhydride | C. tessmannii | Roots | Methanol extract | NMR, UV, IR, MS, | Cameroon | [97] | |
| 204. | Alcohol | 1,2-Epoxylinalool | C. sinensis | Leaves and | SFE-CO2 | GC-MS GC-MS | China | [98] |
| 205. | Alcohol | 1-Eicosanol | C. africana | stems
Leaves |
Ethyl acetate extract | 2D-GC-TOF/MS | South Africa | [27] |
| 206. | Alcohol | 1-Hexacosanol | C. pallida | Aerial parts | Ethanol extract | GC-MS | Mexico | [36] |
| 207. | Alcohol | 1-Hexadecanol | C. sinensis | Leaves and | SFE-CO2 | GC-MS | China | [98] |
| 208. | Alcohol | 1-Propanol, 2-(dimethyl-amino)-2-methyl | C. africana | stems
Fruits |
Hexane extract | 2D-GC-TOF/MS | South Africa | [27] |
| 209. | Alcohol | 1-Tetracosanol | C. pallida | Aerial parts | Ethanol extract | GC-MS | Mexico | [36] |
| 210. | Alcohol | 2,2,3,4-Tetramethylhex-5-en-3-ol | C. sinensis | Leaves and | SFE-CO2 | GC-MS | China | [98] |
| 211. | Alcohol | 2-Ethyl-1-hexanol | C. sinensis | stems
Leaves and |
SFE-CO2 | GC-MS | China | [98] |
| 212. | Alcohol | 2-Hexen-1-ol | C. sinensis | stems
Leaves and |
SFE-CO2 | GC-MS | China | [98] |
| 213. | Alcohol | 2-Methyl-1-hexadecanol | C. sinensis | stems
Leaves and |
SFE-CO2 | GC-MS | China | [98] |
| 214. | Alcohol | 3,4,4-Trimethyl-3-pentanol | C. africana | stems
Fruits |
Ethyl acetate extract | 2D-GC-TOF/MS | South Africa | [27] |
| 215. | Alcohol | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | C. iguanaea | Leaves | Dichloromethane and ethanol | GC-MS | Brazil | [108] |
| 216. | Alcohol | 3,7-Dimethyl-2,6-octadien-1-ol | C. sinensis | Leaves and | extract SFE-CO2 | GC-MS | China | [98] |
| 217. | Alcohol | 3-Hexanol,4,4-dimethyl- | C. africana | stems
Leaves |
Hexane extract | 2D-GC-TOF/MS | South Africa | [27] |
| 218. | Alcohol | 3-Hexen-1-ol | C. sinensis | Leaves and | SFE-CO2 | GC-MS | China | [98] |
| 219. | Alcohol | Docosanol | C. africana
C. pallida |
stems
Leaves Aerial parts |
Hexane extract Ethanol extract | 2D-GC-TOF/MS GC-MS | South Africa Mexico | [27]
[36] |
| 220. | Alcohol | Mome inositol | C. africana | Leaves, | Dichloromethane: methanol extract | 2D-GC-TOF/MS | South Africa | [27] |
| Fruits & | ||||||||
| Stems | ||||||||
site
GC-MS
H-NMR, C-NMR
stems
(continued on next page)
Table 3 (continued )
Compound Number
Chemical class Compound Species Organs
Extract
Structure elucidation Collection
site
Rf no.
- Alcohol ND2H-1-Benzopyran-6-ol,3,4-dihydro-2,7,8- trimethyl- 2-(4,8,12-trimethyltridecyl)
C. africana Fruits Ethyl acetate extracts 2D-GC-TOF/MS South Africa [27]
- Alcohol n-Tridecan-1-ol C. africana Stems Ethyl acetate extract 2D-GC-TOF/MS South Africa [27]
- Alcohol Sapiol C. adolphi- friderici
Roots Acetone extract NMR and MS Cameroon [103]
- Alcohol trans-9-Hexadecen-1-ol C. sinensis Leaves and Stems
- Aldehyde 14-Hexadecenal C. sinensis Leaves and Stems
SFE-CO2 GC-MS China [98]
SFE-CO2 GC-MS China [98]
- Aldehyde 2,4-Heptadienal C. africana Stems Ethyl acetate extract 2D-GC-TOF/MS South Africa [27]
- Aldehyde 2-Heptenal C. africana Fruits Dichloromethane: methanol extract 2D-GC-TOF/MS South Africa [27]
C. africana Stems Ethyl acetate extract 2D-GC-TOF/MS South Africa [27]
- Aldehyde 2-Propylhexanal C. africana Fruits Hexane extract 2D-GC-TOF/MS South Africa [27]
- Aldehyde
3,5-Dihydroxybenzaldehyde
C. australis Fruits &
Leaves
Ethanol extract UHPLC–QqQ-MS/ MS, UV
Croatia [32]
- Aldehyde
4-Hydroxybenzaldehyde
C. tournefortii Fruits, Leaves & Young twigs
Methanol–dichloromethane extract HPLC-TOF/MS Turkey [114]
- Aldehyde Benzaldeyde C. sinensis Leaves and Stems
- Aldehyde Benzeacetaldehyde C. sinensis Leaves and stems
SFE-CO2 GC-MS China [98]
SFE-CO2 GC-MS China [98]
- Aldehyde Deca-2,4-dienal C. africana Fruits Dichloromethane: methanol extract 2D-GC-TOF/MS South Africa [27]
C. africana Stems Ethyl acetate extract 2D-GC-TOF/MS South Africa [27]
- Aldehyde Hexanal C. africana Stems Ethyl acetate extract 2D-GC-TOF/MS South Africa [27]
- Aldehyde
Indole-3-carboxaldehyde
C. adolphi- friderici
Roots Acetone extract Cameroon [103]
- Alkane (—)-trans-Pinane C. zenkeri Leaves Methanol GC-MS Nigeria [107]
- Alkane (R)-1-Methyl-4-(1-methylethyl)-cyclohexene C. sinensis Leaves and
stems
- Alkane 1-Docosene C. sinensis Leaves and stems
- Alkane 1α,2α,4α-1, 2,4-Trimethyl-cyclohexane C. sinensis Leaves and
stems
- Alkane 2,6,10,15-Tetramethyl-heptadecane C. sinensis Leaves and
stems
- Alkane 2,6,10-trimethyl-tetradecane C. sinensis Leaves and
stems
- Alkane 6-Tridecene C. sinensis Leaves and stems
- Alkane 7-Tetradecene C. sinensis Leaves and stems
- Alkane Benzedrex C. sinensis Leaves and stems
- Alkane bicyclohexane C. sinensis Leaves and stems
- Alkane cis-1,2-Dimethyl-cyclohexane C. sinensis Leaves and
stems
SFE-CO2 GC-MS China [98]
SFE-CO2 GC-MS China [98]
SFE-CO2 GC-MS China [98]
SFE-CO2 GC-MS China [98]
SFE-CO2 GC-MS China [98]
SFE-CO2 GC-MS China [98]
SFE-CO2 GC-MS China [98]
SFE-CO2 GC-MS China [98]
SFE-CO2 GC-MS China [98]
SFE-CO2 GC-MS China [98]
(continued on next page)
M.A. Samadd et al.
Heliyon 10 (2024) e29707
20
Table 3 (continued )
Compound Number
Chemical class Compound Species Organs
Extract
Structure elucidation Collection
site
Rf no.
- Alkane cis-1-Ethyl-2-methyl-cyclohexane C. sinensis Leaves and
stems
- Alkane Decane C. sinensis Leaves and stems
- Alkane Dodecane C. sinensis Leaves and stems
- Alkane Ethyl-cyclohexane C. sinensis Leaves and stems
- Alkane Heptadecane C. sinensis Leaves and stems
- Alkane Hexadecane C. sinensis Leaves and stems
- Alkane Nonadecane C. sinensis Leaves and stems
- Alkane Nonane C. sinensis Leaves and stems
- Alkane Octadecane C. sinensis Leaves and stems
- Alkane Pentyl-cyclohexane C. sinensis Leaves and stems
- Alkane Tetradecane C. sinensis Leaves and stems
SFE-CO2 GC-MS China [98]
SFE-CO2 GC-MS China [98]
SFE-CO2 GC-MS China [98]
SFE-CO2 GC-MS China [98]
SFE-CO2 GC-MS China [98]
SFE-CO2 GC-MS China [98]
SFE-CO2 GC-MS China [98]
SFE-CO2 GC-MS China [98]
SFE-CO2 GC-MS China [98]
SFE-CO2 GC-MS China [98]
SFE-CO2 GC-MS China [98]
- Alkene 2,4-Dimethylpenta-1,3-diene C. zenkeri Leaves GC-MS Nigeria [99]
- Alkene 3,5-Dimethyl-1,6-heptadiene C. zenkeri Leaves GC-MS Nigeria [99]
- Alkene Nonadecene C. zenkeri Stem bark GC-MS Nigeria [99]
- Amino acid 2-Aminooctanoic acid C. pallida Aerial parts Ethanol extract GC-MS Mexico [36]
- Amino acid Aspartic acid C. adolphi- friderici
Roots Acetone extract EI-MS, H-NMR, C- NMR
Cameroon [103]
- Benzene 1,2,4,5-Tetramethyl-benzene C. sinensis Leaves and
stems
- Benzene 1,3,5-Trimethyl-benzene C. sinensis Leaves and stems
- Benzene 1,3-Diethyl-benzene C. sinensis Leaves and stems
- Benzene 1,4-Diethyl-benzene C. sinensis Leaves and stems
- Benzene 1-Ethyl-3-methyl-benzene C. sinensis Leaves and stems
- Benzene 1-Isocyano-2-methyl-benzene C. sinensis Leaves and
stems
- Benzene Ethylbenzene C. sinensis Leaves and stems
- Benzene Naphthalene C. sinensis Leaves and stems
- Benzene p-Xylene C. sinensis Leaves and stems
SFE-CO2 GC-MS China [98]
SFE-CO2 GC-MS China [98]
SFE-CO2 GC-MS China [98]
SFE-CO2 GC-MS China [98]
SFE-CO2 GC-MS China [98]
SFE-CO2 GC-MS China [98]
SFE-CO2 GC-MS China [98]
SFE-CO2 GC-MS China [98]
SFE-CO2 GC-MS China [98]
- Benzopyrone Scopoletin C. laevigata Leaves Aqueous extract UV, Chromatographed
M.A. Samadd et al.
Heliyon 10 (2024) e29707
21
United States [126] (continued on next page)
Table 3 (continued )
M.A. Samadd et al.
Heliyon 10 (2024) e29707
22
| Compound Number | Chemical class | Compound | Species | Organs | Extract | Structure elucidation | Collection | Rf no. |
| 273.
274. |
Benzopyrone glycoside Dimer | Scopolin
Quinic acid-O- Malic acid |
C. laevigata
C. eriocarpa |
Leaves
Leaves |
Aqueous extract
Methanol extract |
UV,
UHPLC-DAD, ESI-MS |
United States
Pakistan |
[126]
[118] |
| 275. | Dimer | Quinic acid-O-tartaric acid | C. eriocarpa | Leaves | Methanol extract | UHPLC-DAD, ESI-MS | Pakistan | [118] |
| 276. | Hydroxy pyrone | 3-hydroxy-2-methyl-4H-pyran-4-one | C. africana | Fruits | Dichloromethane: methanol extract | 2D-GC-TOF/MS | South Africa | [27] |
| 277. | Ketone | 2-Pyrrolidinone, 1-methyl- | C. zenkeri | Leaves | Methanol extract | GC-MS | Nigeria | [107] |
| 278. | Ketone | 1-(4-hydroxy-3-methoxyphenyl) ethanone | C. africana | Stems | Dichloromethane: methanol extract | 2D-GC-TOF/MS | South Africa | [27] |
| 279. | Ketone | 2,3-Heptanedione | C. africana | Stems & | Hexane extract | 2D-GC-TOF/MS | South Africa | [27] |
| 280. | Ketone | 2,3-Pentanedione | C. africana | Leaves
Fruits, |
Hexane extract | 2D-GC-TOF/MS | South Africa | [27] |
| 281. | Ketone | 3,4-Dimethyldihydrofuran-2,5-dione | C. africana | Leaves &
Stems |
Hexane extract | 2D-GC-TOF/MS | South Africa | [27] |
| 282. | Ketone | 3,5,6,7,8,8a-hexahydro-4,8a-dimethyl-6-(1- | C. africana | Stems | Dichloromethane: methanol extract | 2D-GC-TOF/MS | South Africa | [27] |
| 283. | Ketone | methylethenyl- 2(1H)naphthalenone 3-Hydroxy-5,6-epoxy-aˆ-ionone | C. sinensis | Leaves and | SFE-CO2 | GC-MS | China | [98] |
| 284. | Ketone | Cyclohexanone | C. sinensis | stems
Leaves and |
SFE-CO2 | GC-MC | China | [98] |
| 285. | Ketone | Jasmone | C. sinensis | stems
Leaves and |
SFE-CO2 | GC-MS | China | [98] |
| 286. | Lignan glycoside | Pinoresinol-4-O-glucoside | C. sinensis | stems
Twigs |
Methanol extract | H-NMR, C-NMR, FT- | Korea | [101] |
| 287. | Lignan glycoside | Pinoresinol-4-O-rutinoside | C. sinensis | Twigs | Methanol extract | IR, UV
H-NMR, C-NMR, FT- |
Korea | [101] |
| 288. | Lipid | 1-O-(β-D-glucopyranosyl) -(2S,3S,4R,5E)-2N- | C. africana | Aerial parts | Ethanol-water extract | IR, UV
2D-NMR, MS |
Saudi Arabia | [37] |
| (glucosphingolipid) | ([2′R,6′E]-2′-hydroxyoctadeca-6′-enoylamino)- | |||||||
| 289.
290. |
Lipid (glucosphingolipid) Nitrogeneous base | 5-pentadecaene-1,3,4-triol
Eloundemnoside 2-Amino-9-(3,4-dihydroxy-5-hydroxymethyl- |
C. adolphi- friderici
C. africana |
Roots
Leaves, |
Acetone extract
Dichloromethane: methanol |
H-NMR, C-NMR,
2D-GC-TOF/MS |
Cameroon
South Africa |
[103]
[27] |
| tetrahydrofuran-2-yl)-3,9 dihydro-purin-6-one | Fruits & | |||||||
| 291. Nitrogeneous base | 2,4(1H,3H)-pyrimidinedione,5-methyl | C. africana | Stems | Dichloromethane: methanol | 2D-GC-TOF/MS | South Africa | [27] | |
| 292. Phenol | 2,2′-Methylenebis[6-(1,1-dimethylethyl)-4- | C. sinensis | Leaves and | SFE-CO2 | GC-MS | China | [98] | |
| 293. | Phenol | methyl]-phenol
2,4-bis(1,1-dimethylethyl)-Phenol |
C. sinensis | stems
Leaves and |
SFE-CO2 | GC-MS | China | [98] |
| 294. | Phenol | 2-Methoxy-6-(2-propenyl)-phenol | C. sinensis | stems
Leaves and |
SFE-CO2 | GC-MS | China | [98] |
| 295. | Phenol | 5-Pentyl-1,3-benzenediol | C. sinensis | stems
Leaves and |
SFE-CO2 | GC-MS | China | [98] |
| 296. | Phenol | Butylated hydroxytoluene | C. sinensis | stems
Leaves and |
SFE-CO2 | GC-MS | China | [98] |
| 297. | Phenol | Eugenol | C. sinensis | stems
Leaves and |
SFE-CO2 | GC-MS | China | [98] |
| 298. Phenolic aldehyde | Ferulaldehyde | C. eriocarpa | stems
Leaves |
Methanol extract | UHPLC-DAD, ESI-MS | Pakistan | [118] | |
| 299. Steroid | (3β,9β,14β)-14-hydroxy-9,19-cyclocholan-3-yl | C. australis | Stem barks | Ethanol extract | H-NMR, C-NMR, IR, | India | [119] | |
| β-D-glucopyranoside | & Fruits | MS | ||||||
site
Chromatographed
Stems
HRESIMS, UV, IR
Stems
(continued on next page)
Table 3 (continued )
Compound Number
Chemical class Compound Species Organs
Extract
Structure elucidation Collection
site
Rf no.
- Sterol α-Sitosterol C. africana Fruits Dichloromethane: methanol extract 2D-GC-TOF/MS South Africa [27]
C. africana Stems &
Leaves
C. sinensis Leaves and stems
Hexane extract 2D-GC-TOF/MS South Africa [27]
SFE-CO2 GC-MS China [98]
- Sterol β-sitosterol C. adolphi-
friderici
Roots Acetone extract H-NMR, C-NMR, HRESIMS, UV, IR
Cameroon [103]
C. africana Aerial parts Ethanol-water extract H-NMR, C-NMR,
EIMS, HREIMS
Saudi Arabia [102]
C. australis Leaves Hydro-methanolic extract H-NMR, C-NMR, Morocco [127]
C. pallida Aerial parts Ethanol extract GC-MS Mexico [36]
C. sinensis Twigs Methanol extract H-NMR, C-NMR, FT- IR, UV
C. tessmannii Stem barks Methanol extract NMR, UV, IR, MS, GC-MS
C. zenkeri Stem barks Methanol HREIMS, H-NMR, C- NMR
Korea [101]
Cameroon [97]
Nigeria [90]
- Sterol Gamma-sitosterol C. ehrenbergiana Leaves Crude methanolic extract GC-MS Brazil [109]
C. iguanaea Leaves Dichloromethane & ethanol extract GC-MS Brazil [108]
- Sterol glycoside β-sitosterol-3-O-β-glucoside C. australis Leaves Hydro-methanolic extract H-NMR, C-NMR, Morocco [127]
C. sinensis Twigs Methanol extract H-NMR, C-NMR, FT- IR, UV,
Korea [101]
- Sterol glycoside β-sitosterol-3-O-β-D-glucopyranoside C. adolphi-
friderici
Roots Acetone extract Cameroon [103]
C. zenkeri Stem bark Methanol extract HREIMS, H-NMR, C- NMR
Nigeria [90]
- Stilbene Resveratrol C. tournefortii Fruits Water, ethanol and methanol extract
RP-HPLC-DAD, UV Turkey [33]
C. tournefortii Leaves Methanol–dichloromethane extract HPLC-TOF/MS Turkey [114]
- Stilbenoid glycoside Polydatine C. tournefortii Fruits, Leaves & Young twigs
Methanol–dichloromethane extract HPLC-TOF/MS Turkey [114]
- Sugar cis-1-O-methylinositol C. tessmannii Roots Methanol extract NMR, UV, IR, MS,
GC-MS
Cameroon [97]
- Sugar Sucrose C. pallida Aerial parts Ethanol extract GC-MS Mexico [36]
- Sugar D-Turanose C. pallida Aerial parts Ethanol extract GC-MS Mexico [36]
- Tannin Glucosyringic acid C. tessmannii Roots Methanol extract NMR, UV, IR, MS,
GC-MS
Cameroon [97]
- Tannin Ellagic acid C. iguanaea Leaves 70 % ethanol HPLC Brazil [112]
C. tournefortii Fruits Methanol extract HPLC, UV Turkey [113]
C. tournefortii Leaves &
young twigs
Methanol–dichloromethane extract HPLC-TOF/MS Turkey [114]
M.A. Samadd et al.
Heliyon 10 (2024) e29707
23
terpenoids, alcohol, esters, and sterol [108] as well as flavonoids [121]. However, only flavonoid compounds were reported in the leaves of C. choseniana [68] and the barks of C. tetrandra [116]. The twigs of C. philippinensis contain several terpenoids and terpenoid esters [115]. Phytochemical analysis of the methanol extract of C. eriocarpa leaves revealed the existence of acid anhydrate, fatty acids, phenolic acids, esters, flavonoids, and glycosides [118]. In addition to phenolic acids, benzopyrone and glycoside were isolated from the leaves of C. laevigata as chief cytotoxic compounds [126]. Amides, terpenoids, sterols, and glycosides are also present in the twigs of
C. sinensis [101]. Furthermore, amides, terpenoids, and sterols were also discovered in the stem bark of C. tessmannii, along with acid anhydrate, tannin, and sugar in the roots [97].
Phytochemicals (primary and secondary metabolites) have been known for their wide range of therapeutic advantages for plants and humans [130]. Plant metabolic reactions such as photosynthesis and respiration are controlled by primary metabolites such as chlorophyll, lipids, carbohydrates, proteins, nucleic acids, and amino acids [95,131,132]. Secondary metabolites include terpenoids, flavonoids, alkaloids, phenols, saponins, tannins, steroids, and glycosides, all of which play critical roles in shielding plants from degradation and boosting plant fragrance, appearance and texture [95,132].
Numerous molecules from these classes have been found and evaluated for pharmacological effects in Celtis plants. Despite the
ample papers on phytochemical analysis among many species of Celtis, the structure identification procedure of molecules from these species needs to be explicitly stated in all articles. The compounds were identified using two dimensional time of flight mass spec- troscopy (2D-GC-TOF/MS), proton nuclear magnetic resonance (1H-NMR), carbon-13 nuclear magnetic resonance (13C-NMR), elec-
trospray ionization mass spectrometry (ESI-MS), high-resolution fast atom bombardment mass spectroscopy (HR-FAB-MS), gas chromatrography mass spectrometry (GC-MS), reversed-phase high performance liquid chromatography (RP-HPLC), triple quadrupole mass spectroscopy (QqQ-MS), infrared (IR), reversed-phase high performance liquid chromatography diode-array detection (RP- HPLC-DAD), liquid chromatography mass spectrometry (LC-MS), ultra high-pressure liquid chromatography diode-array detection (UHPLC-DAD), high-resolution electron ionization mass spectrometry (HREIMS), high-resolution electrospray ionization mass spec- trometry (HRESIMS), flame ionization detector (FID), and ultra high-pressure liquid chromatography-orbitrap mass spectrometry (UHPLC-Orbitrap-MS).
Till now, very few amide compounds have been found in Celtis species plants. The majority of them are hydroxycinnamic acid derivatives (compounds 4–10), with only two being ceramides (compounds 1–2) (Table 3) (Fig. 1). Hydroxycinnamic acid derivatives are primarily found in aerial parts, roots, and twigs of Celtis plants, while ceramides are present in the stem barks only. The frequently
reported amides in Celtis species is compound 8, which is obtained from the aerial parts of C. africana [102], roots of C. adolphi-friderici
[103], stem barks of C. zenkeri and C. tessmannii [90,97], and twigs of C. sinensis and C. occidentalis [100,101]. Two new ceramides compounds 1–2 were detected as pure compounds from a methanol extract of C. tessmannii stem barks using NMR, ultra violet spectroscopy (UV), IR, MS, and GC-MS methods [97], while a noble iso-benzo-furanone propenamide (compound 11) was discovered from Central African plant C. zenkeri [90]. Compound 3 is the only fatty acid derivative amide found in this study noted from the supercritical fluid extraction of carbon di-oxide (SFE-CO2) extraction of C. sinensis leaves and stems and leaves of C. zenkeri [98,99]. All
the amide compounds obtained from the genus Celtis are sketched in Fig. 2.
In addition to organic acids, various ester compounds such as carboxylic esters (compounds 13–16), fatty esters (compounds 18–50), and triterpene esters (compounds 53–54) have been documented from Celtis plants (Table 3). These constituents are mainly identified in leaves, fruits, and stems. Compounds 53 and 54 (triterpene esters) are found for the first time in a methanolic extract of
C. philippinensis twigs through fourier-transform infrared spectroscopy (FT-IR), HR-FAB-MS, H-NMR, and C-NMR techniques [115]. Most isolated esters from the Celtis plants come from a species, but some molecules, including compounds 27, 40, 41, 43, 45, and 51,
have been detected from more than one species (Table 3). Among them, only the compound 51 has been noted in three different species, including C. australis, C. iguanaea, and C. tournefortii [111–113]. Various types of fatty acid esters are reported from the ripe fruits of C. australis via the GC-MS method, where methyl ester of these fatty acids is the most dominating compound (71.60 %) of the total fatty acid composition (95.45 %) [31]. Another compound 49 was the main identified compound among the seventy-three
different identified volatile compounds of C. sinensis, isolated from both leaves and stems at 20.79 % and 23.76 % of total contents, respectively [98]. The only isolated anthraquinone ester of these species, compound 12 is reported from an Indian study of C. australis stem barks and fruits using H-NMR, C-NMR, IR, and MS techniques [105]. Solely phenolic ester, compound 52, is detected from fruits, leaves, and young twigs of C. tournefortii via HPLC-TOF/MS methods [114], while the only steroid and fatty acid derivative ester, compound 50, is reported from crude methanolic extract of C. ehrenbergiana through GC-MS manner [109]. A Cameroonian investi- gation of acetone extract of C. adolphi-friderici roots isolated 3.3 mg of compound 27, the only reported ester of this species [103]. All the organic esters compounds obtained from the genus Celtis are sketched in Fig. 3.
Flavonoids are the most documented components in Celtis species classified into flavanonol (compound 71), flavanol (compounds 58–64), flavonol (compounds 110–120), flavone (compounds 72–109), flavanone (compounds 65–70) , and anthocyanins (com- pounds 55–57) (Table 3). Leaves and fruits are the main reservoirs of these molecules, but they are also detected in the bark, young
twigs, and aerial parts (Table 3). Compound 59 is the most frequently reported flavanol in the Celtis species, isolated from various parts of C. pallida, C. tetrandra,and C. tournefortii [113,114,116,117], while the other flavanols are mainly epimers of compounds 58 and 59 (Table 3). However, compounds 108, 115, and 120 are the most commonly detected flavonoid molecules of the Celtis genus isolated from the various extracts of different parts of the five distinct species (Table 3). Compound 105, a flavone glycoside, was also isolated from four distinct Celtis species (C. africana, C. australis, C. occidentalis, and C. iguanaea) [121–123]. Among the conjugate molecules of
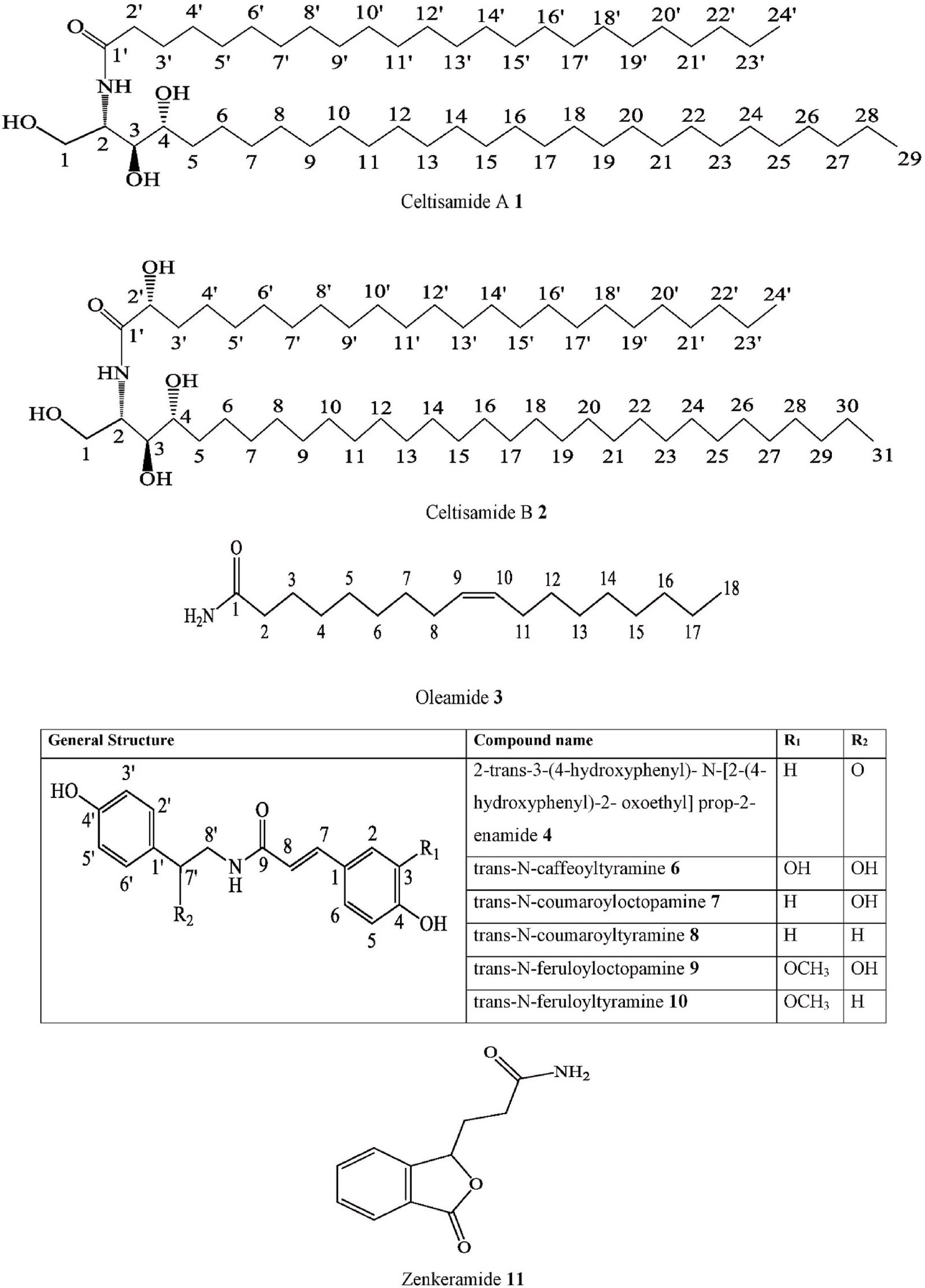
Fig. 2. Amides from the genus Celtis.
flavonoids, flavones and flavanone glycosides are the most extracted compounds (Table 3), while two new flavanol dimers (com-
pounds 63, and 64) are identified from the ethyl acetate extract of C. tetrandra barks using MS, H-NMR, C-NMR, and HRESIMS [116]. Ten flavonoids’ glycosides are attributed to compound 108 and its derivatives, discovered in the five following different species,
C. africana, C. australis, C. eriocarpa, C. occidentalis, and C. iguanaea [111,118,121–123]. In this study, all flavonoids of C. occidentalis
[118,122] and almost all of C. eriocarpa were identified as glycoside compounds, while most of the C. ericocarpa flavonoids were the
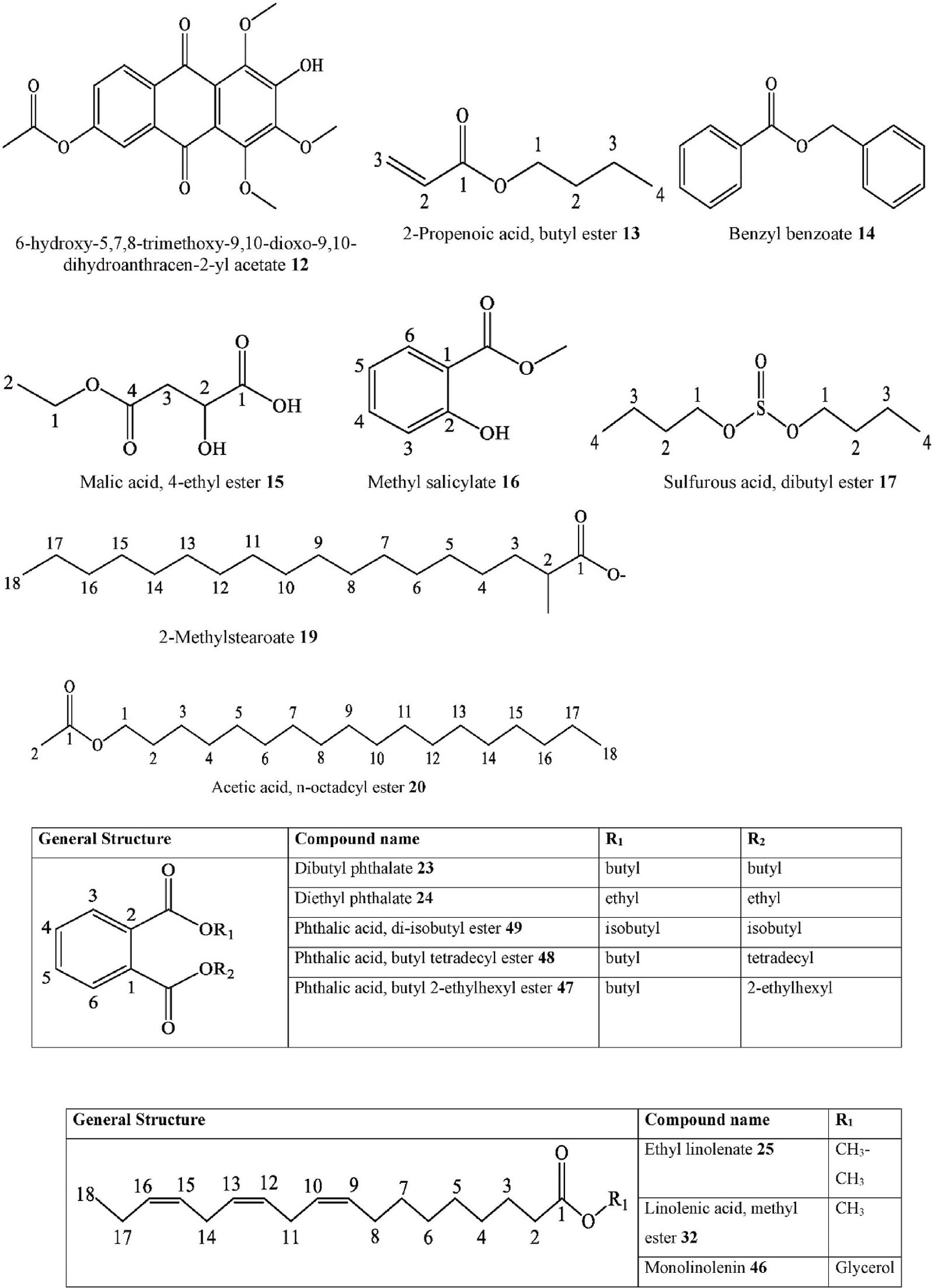
Fig. 3. Esters from the genus Celtis.
Fig. 3. (continued).
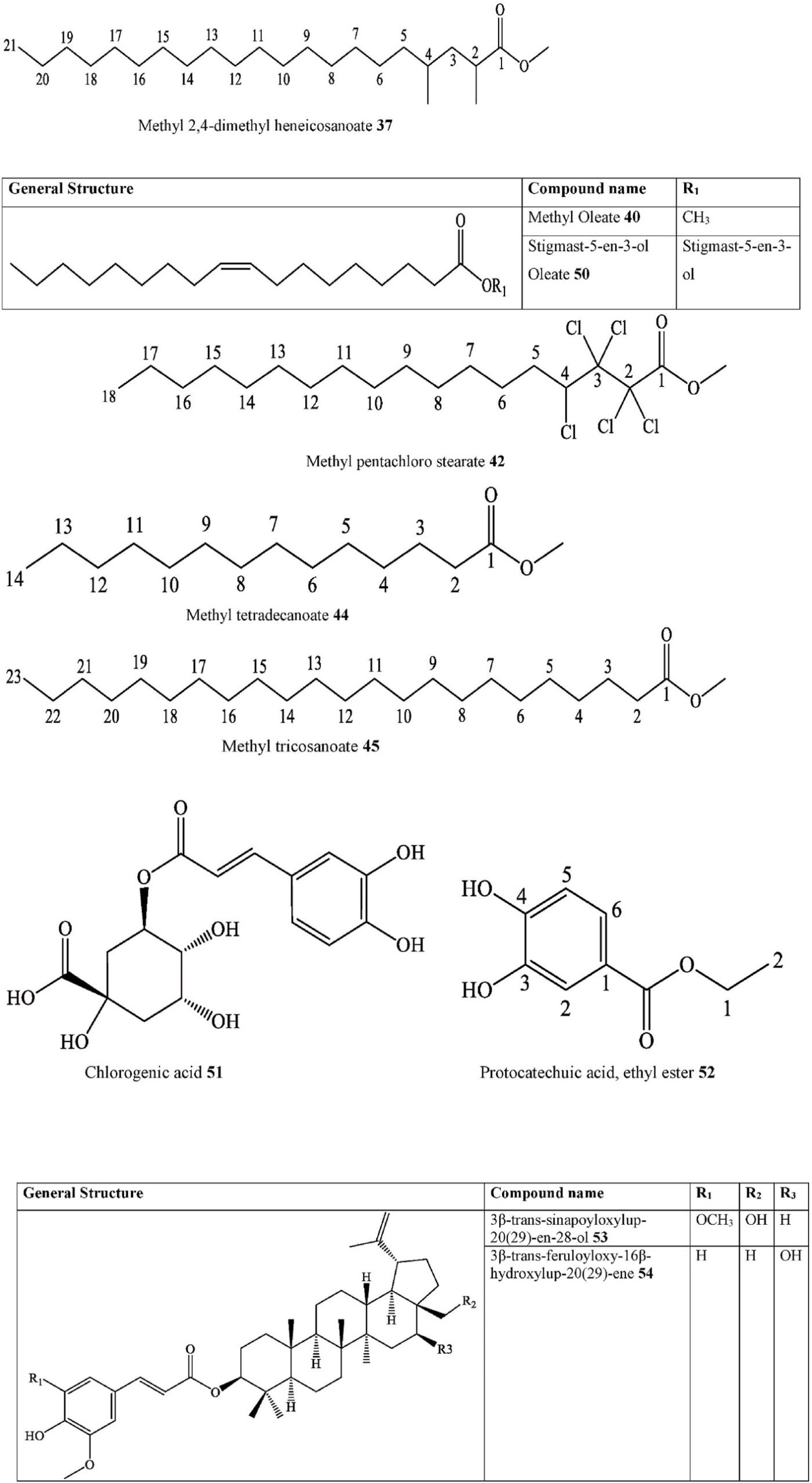
Fig. 3. (continued).
first time reported molecules [118]. Three different anthocyanins have been reported from the fruits and leaves of C. australis [32]. A
new C-triglycoside, compound 83, is obtained from the leaves of C. australis and C. occidentalis, whereas compound 81 is the primary isolated component of the n-butanol fraction of the same species’ leaves [122]. Compounds 94 and 95, two novel C-glycosylflavonoids, were discovered in ethanol and water extracts of C. africana aerial parts using HR-FAB-MS, H NMR, C-NMR, GC-MS, and EI-MS techniques [123]. All the organic acid compounds obtained from the genus Celtis are sketched in Fig. 4.
Among the various types of phytochemical molecules of Celtis species, organic acids and their derivatives are the second most reported compounds that can be divided into phenolic acids (compounds 167–172), hydroxycinnamic acids and glycoside (com- pounds 157–166), benzoic acids and derivatives (compounds 132–133, 135–138), fatty acids (compounds 139–156), as well as aliphatic carboxylic acids (compounds 124–131) (Table 3). Most of these compounds were identified in the aerial parts, roots, fruits, and leaves of Celtis plants. The most frequently documented organic acid in the Celtis species is compound 154, which is obtained from
various parts of six different species, including C. tournefortii, C. africana, C. australis, C. pallida,C. ehrenbergiana, and C. sinensis [27,32, 33,36,98,102,109]. Furthermore, compound 156 is reported from four different Celtis species namely: C. australis, C. pallida, C. sinensis, and C. tournefortii [32,33,36,98], while compound 153 is noted from three distinct species: C. africana,C. australis, and C. tournefortii [32,33,102].Compound 148 is extracted from hexane, ethyl acetate, and dichloromethane: methanol extract of C. africana leaves, fruits, and stems [27], while compound 145 is isolated from the only ethanol-water extract of aerial parts of the same species [102].
Along with compounds 139 and 143, 150–151, two different types of fatty acids are identified through UHPLC-DAD and ESI-MS
techniques from the crude methanolic extract of leaves of C. eriocarpa [118]. Seven fatty acids (compounds 140, 142, 146, 149, 148, 152, and 154) were reported from the ethanol-water extract of C. pallida aerial parts via the GC-MS method [36]. A saturated fatty acid compound 155, was solely isolated from the fruits of C. tournefortii [33].
In addition, six distinct phenolic acids (compounds 167–172), methanolic extracts of leaves of C. eriocarpa represent about 40 % of
the total reported hydroxycinnamic acids among the Celtiss pecies (compounds 158, 161, 163, and 164) [118]. Among the hydrox- ycinnamic acids, compound 159 is the most frequently isolated acid of the Celtis genus and was reported from three distinct species (C. australis, C. laevigata, C. pallida, andC. tournefortii) [78,113,114,126]. Two hydroxycinnamic acid compounds, 162 and 165, were
discovered using high performance liquid chromatography and time of flight mass spectrometry (HPLC-TOF/MS) techniques in methanol–dichloromethane extracts of C. tournefortii leaves and young twigs [114]. Compound 166 is solely reported from the fruits of
C. tournefortii [33]. Besides various kinds of organic acids, different types of aliphatic carboxylic acids (compounds 124–131) were
separated from five individual species (C. adolphi-friderici, C. eriocarpa, C. ehrenbergiana, C. tessmannii, and C. tournefortii) through various mass spectrometry techniques in different types of alcoholic or acetone extracts [36,97,103,109,118]. Compound 134 is the only carboxylic acid metabolite noted in this study, which was isolated from acetone extract of roots of C. adolphi-friderici [103]. All the organic acid compounds obtained from the genus Celtis are sketched in Fig. 5.
The plants of the Celtis genus are also documented to possess a variety of terpenoid molecules (Table 3). Most of these molecules were discovered in aerial parts, barks, fruits, leaves, stem barks, and twigs. Among them, triterpenoids (compounds 184–201) are the most dominating terpenoids, with two of their esters (compounds 53–54). Apart from triterpenoids, compounds 178–179 are diter- penes, while the remainders are carotenoids (compounds 175–177) and tocopherols (compounds 180–183) (Table 3). Compound 181 is the often-reported terpene among the Celtis plants, detected in five individual species such as, C. africana,C. australis,
C. ehrenbergiana,C. pallida,and C. tournefortii [27,32,33,36,109]. However, among the triterpenoids, compound 193 is the most re- ported compound that has been found in four individual plants, including C. adolphi-friderici, C. africana,C. tessmannii, and C. iguanaea
[27,97,103,129]. Along with three triterpenoids (compounds 184–186), a triterpenoid glycoside, compound 201, was identified in the ethanol extract of C. australis [105]. Several derivatives (compounds 188–190) were recorded from Celtis species, while compound 191 was found in from methanolic extracts of stem barks of C. tessmannii [97], and the rest of compounds 188–190 were isolated from
C. philippinensis twigs and characterized via NMR and MS techniques [115]. Compound 194 is identified from the various extracts of
C. africana stems [27], while its epimer, compound 192, is reported from twigs of C. sinensis [101] as well as the barks of C. iguanaea [129]. Among the triterpenoids, compound 199 and 200 are found in three distinct species (Table 3), while diterpene compound 178 is also revealed in four different species (C. africana,C. iguanaea,C. pallida,and C. zenkeri) [27,36,99,108]. A novel bacteriohopanoid compound 174 has been isolated from the ethanol extract of C. australis bark [128]. Three different carotenoids (compounds
175–177), were isolated from the Croatian study on fruits of C. australis, where compounds 175 and 176 are two isomers [32]. The
compound 179, a derivative of compound 177 [133], has been identified in the fruits of C. tournefortii [33]. Along with compound 181, three different tocopherols were detected in Celtis plants, including C. africana, C. australis, and C. tourneforttii [27,32,33]. However, terpenoids were not reported from several Celtis species. Thus, further research is necessary to identify more terpenoid derivatives from other Celtis plants (Table 3). All the terpenoid compounds obtained from the genus Celtis are sketched in Fig. 6.
Along with amides, organic acids, terpenoids, flavonoids, and esters, Celtis species have been found to possess a variety of addi- tional volatile chemicals such as acid anhydrates, lipids, aldehydes, esters, alkanes, benzopyrone, ketones, alcohols, sterols, tannins,
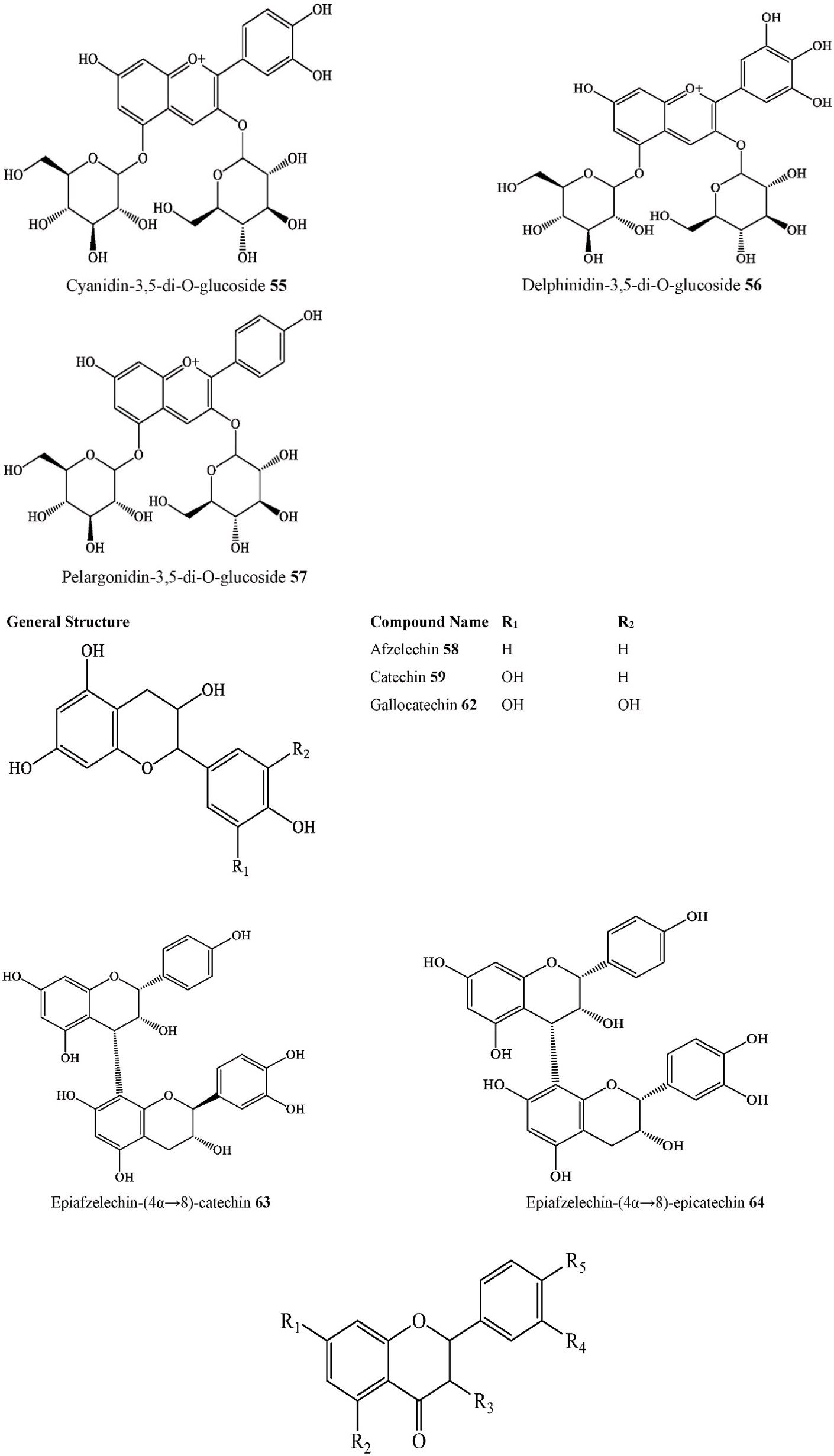
Fig. 4. Flavonoids from the genus Celtis.
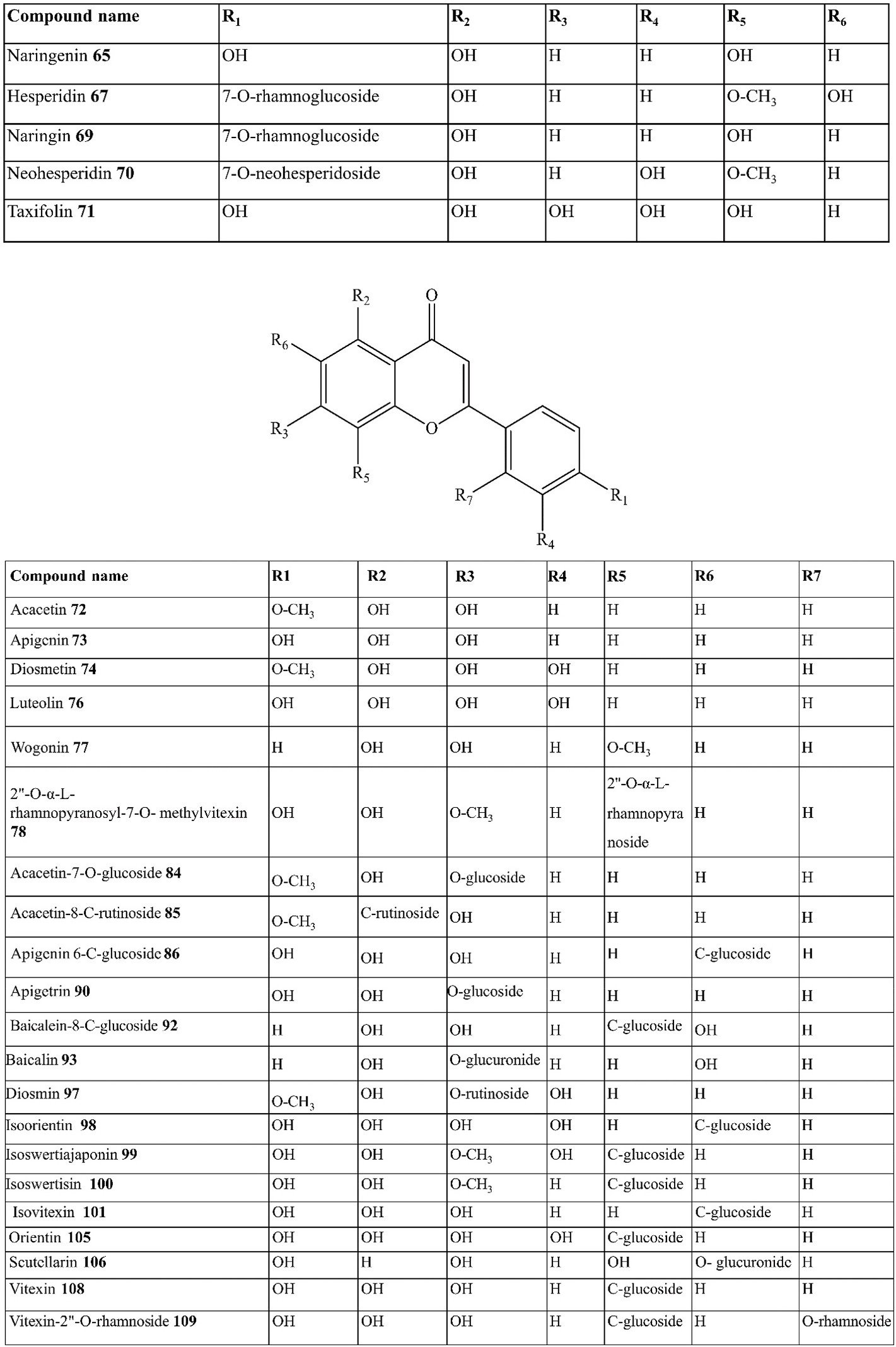
Fig. 4. (continued).
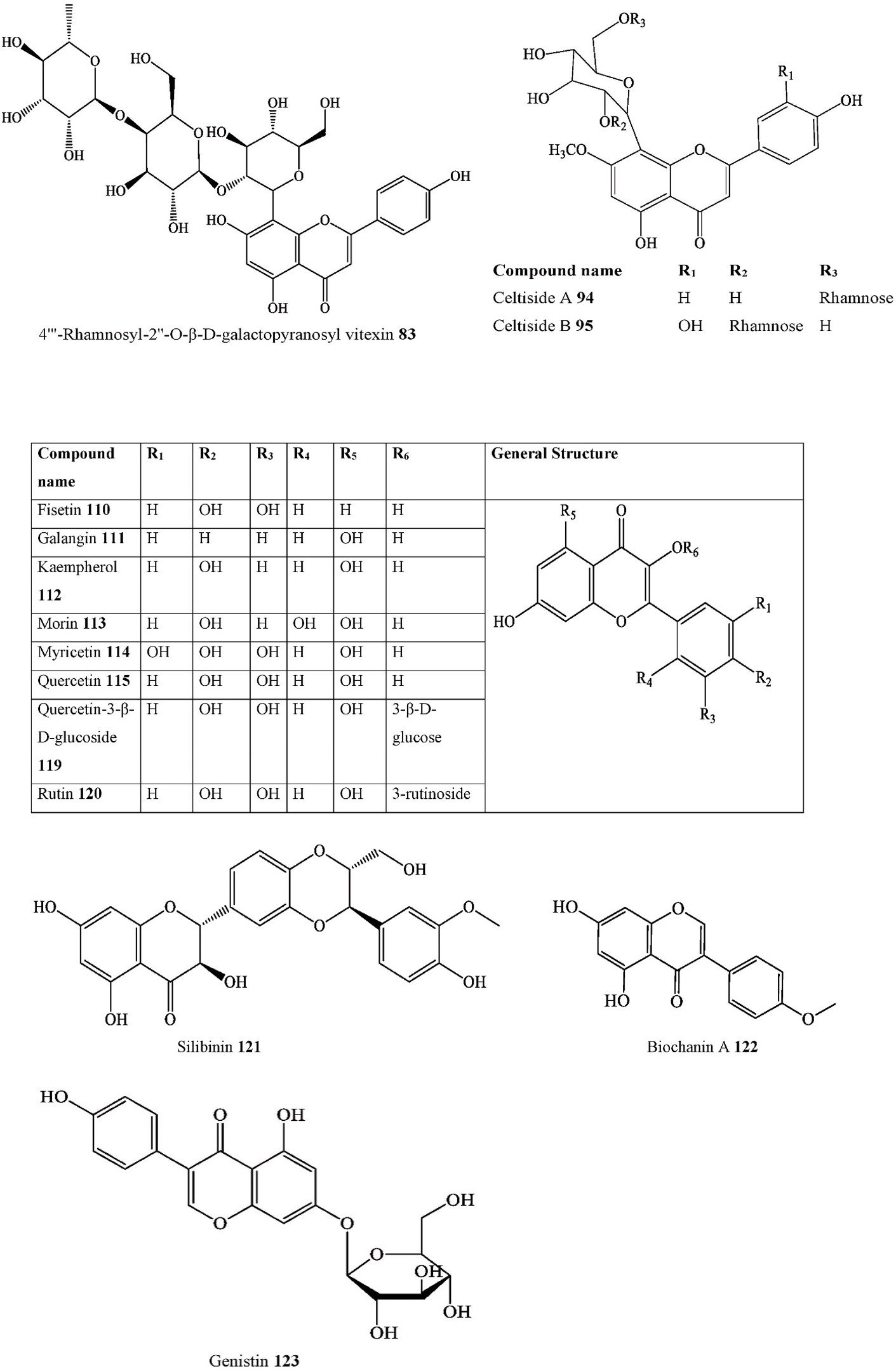
Fig. 4. (continued).
sugars, and others (Table 3). These chemicals have been found in various plant parts, including leaves, fruits, stems, roots, twigs, and aerial parts. Many of these compounds are identified through phytochemical detection using different spectroscopic methods. Almost all aldehydes and ketone molecules from C. africana have been determined using 2D-GC-TOF/MS [27], while alcohol, sterol, sugar, and
amino acid of C. pallida were detected by GC-MS techniques [36]. The majority of the alcohol molecules have been identified from three species: C. pallida, C. africana, and C. sinensis [27,36,98]. In addition to two sterol glycosides, compounds 303–304, three in- dividual sterols, compounds 300–302, are noted from Celtis plants, while the compound 301 is the dominating among them, is found in seven different species (C. africana, C. australis, C. sinensis, C. tessmannii, C. adolphi-friderici, C. zenkeri, and C. pallida) [36,97,101–103, 127,134]. A cytotoxic novel glucosphingolipid, compound 288, is detected from the ethanol-water extracts of C. africana aerial parts
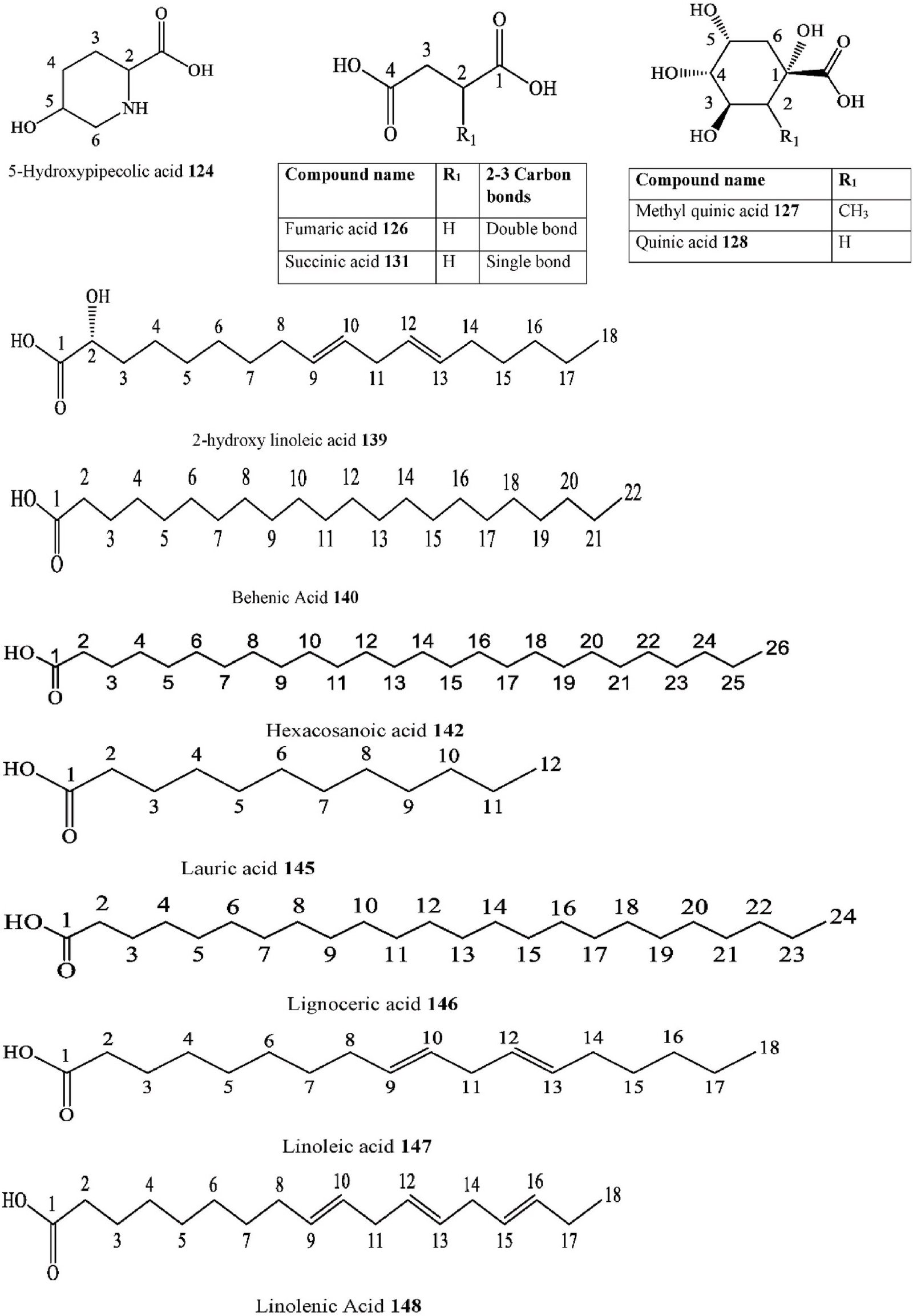
Fig. 5. Organic acids from the genus Celtis.
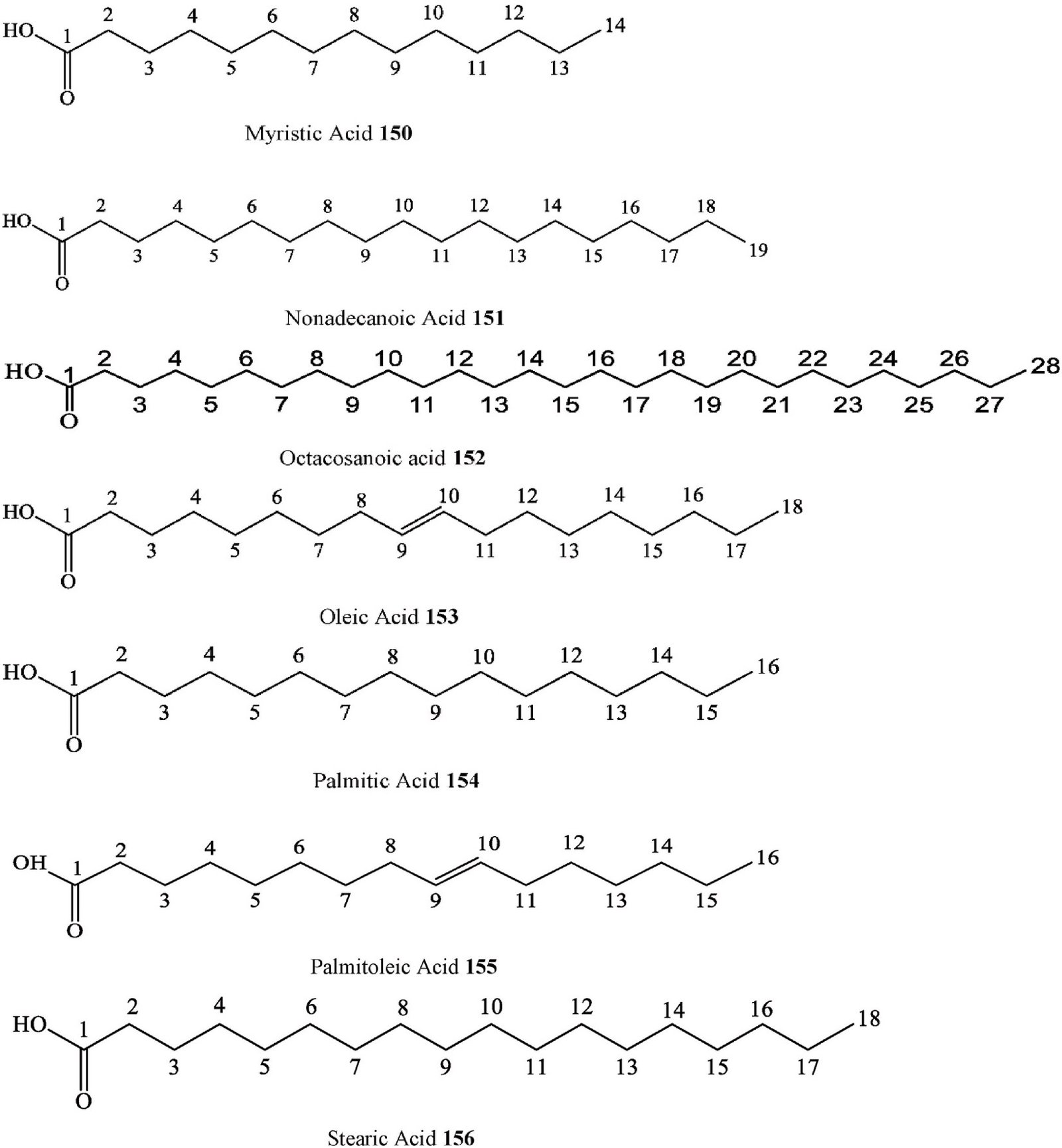
Fig. 5. (continued).
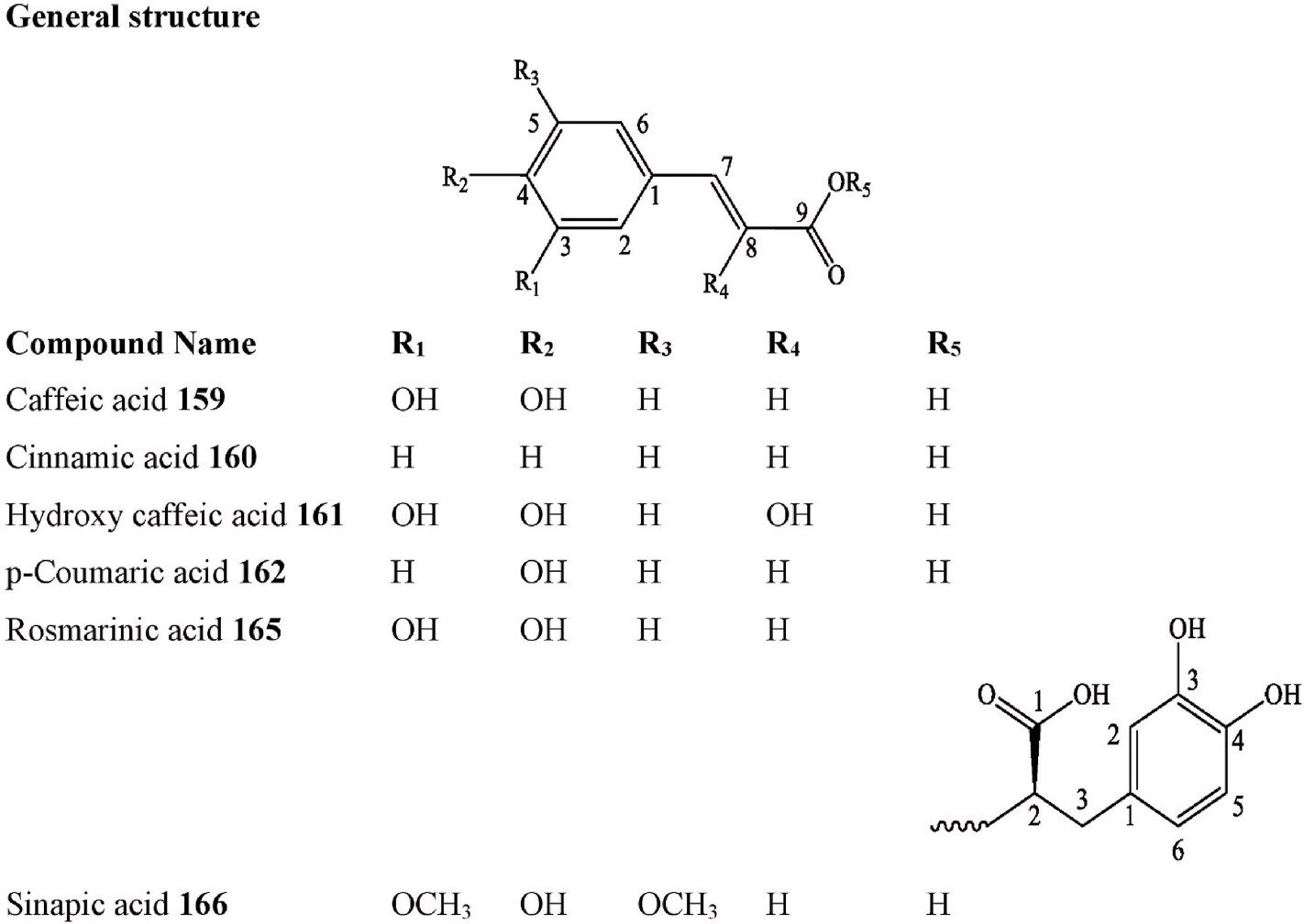
Fig. 5. (continued).
[37], whereas another glucosphingolipid, compound 289, is identified from acetone extracts of C. adolphi-friderici roots through H-NMR, C-NMR, HRESIMS, UV, IR techniques [103]. Moreover, some minor compounds, including tannin, sugar, stilbene, nitrogenous base, lignan, and benzopyrone, are also identified in Celtis plants (Table 3). Two phytotoxic benzopyrones, compounds 272 and 273, are documented in the aqueous extract of C. laevigata leaves [126]. The miscellaneous compounds found from the genus Celtis are sketched in Fig. 7.
Biological activities
Numerous bioactive constituents such as amides, organic acids, terpenoids, flavonoids, ester and several compounds present in Celtis species may account for their various health benefits, and therefore responsible for the vast pharmacological properties (Tables 4 and 5). However, only few species have been extensively studied for bioactivities.
Based on the Antimicrobial Resistance Collaborators study, the six bacteria pathogens causing resistance-related mortality, including Acinetobacter baumannii, Escherichia coli, Staphylococcus aureus, Streptococcus pneumoniae, Klebsiella pneumoniae, and Pseu- domonas aeruginosa, were responsible for 929,000 AMR-related deaths, while total AMR deaths in 2019 were 3.57 million [39]. This figure was higher than the mortality from AIDS and malaria [145]. However, the antibacterial capabilities of medicinal plants provide a possible option for addressing the growing challenges of AMR [40].
The species of Celtis genus may play some significant role because various parts of many Celtis species demonstrated a prominent antimicrobial activity, especially against S. aureus and P. aeruginosa (Table 4). Enormous reports have been noted on the antimicrobial activity of various parts of C. australis such as leaves [32,118,138], ripe fruits [31], and seeds [138]. Dichloromethane extracts of
C. laevigata demonstrated antimicrobial activity against two Mycobacteria organisms, including Mycobacterium tuberculosis and Mycobacterium avium, which were more active against Mycobacterium tuberculosis than Mycobacterium avium [35]. The ethanol extracts of aerial parts of C. pallida were tested against several types of bacteria (Escherichia coli, S. aureus, Bacillus subtilis, and P. aeruginosa), and fungus (Candida albicans); and showed low anti-microbial activity compared with the standard (Cefotaxime) [36].
In C. africana, various extracts of leaves, fruits, and stems exhibit tremendous antimicrobial activity against 2 gram-positive (Bacillus cereus and S. aureus) as well as 5 gram-negative bacteria (Klebsiella pneumoniae,Enterobacter aerogenes,P. aeruginosa,Proteus mirabilis, and E. coli). Among them, high potency was recorded from the hexane extract of fruits against S. aureus, while ethyl acetate extract of stems demonstrated a mild growth inhibitory effect against K. pneumonia, P. aeruginosa, S. aureus, P. mirabilis, and E. coli [27]. Hexane extracts of fruits and leaves demonstrated mild potency against only two organisms E. aerogenes and P. aeruginosa [27]. However, these extracts did not exhibit any activity against M. tuberculosis,B. subtilis,Klebsiella oxytoca,Enterobacter cloacae,Proteus vulgaris, and Staphylococcus epidermidis [27]. Intriguingly, an acetone extract of leaves of C. africana also showed potent antifungal activity against Cryptococcus neoformans (Minimum inhibitory concentrations (MIC) 0.22 mg/ml) [28].
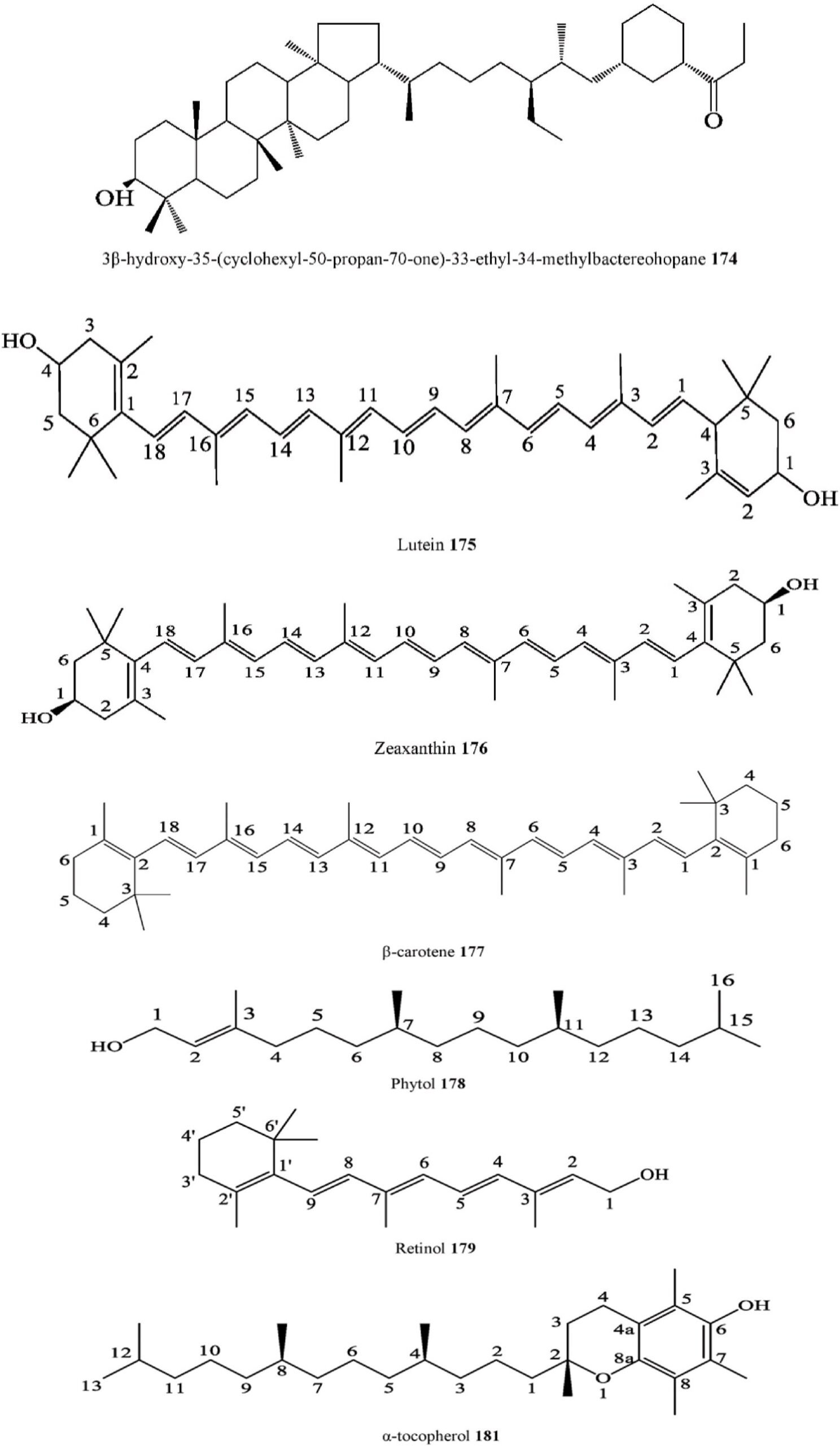
Fig. 6. Terpenoids from the genus Celtis.
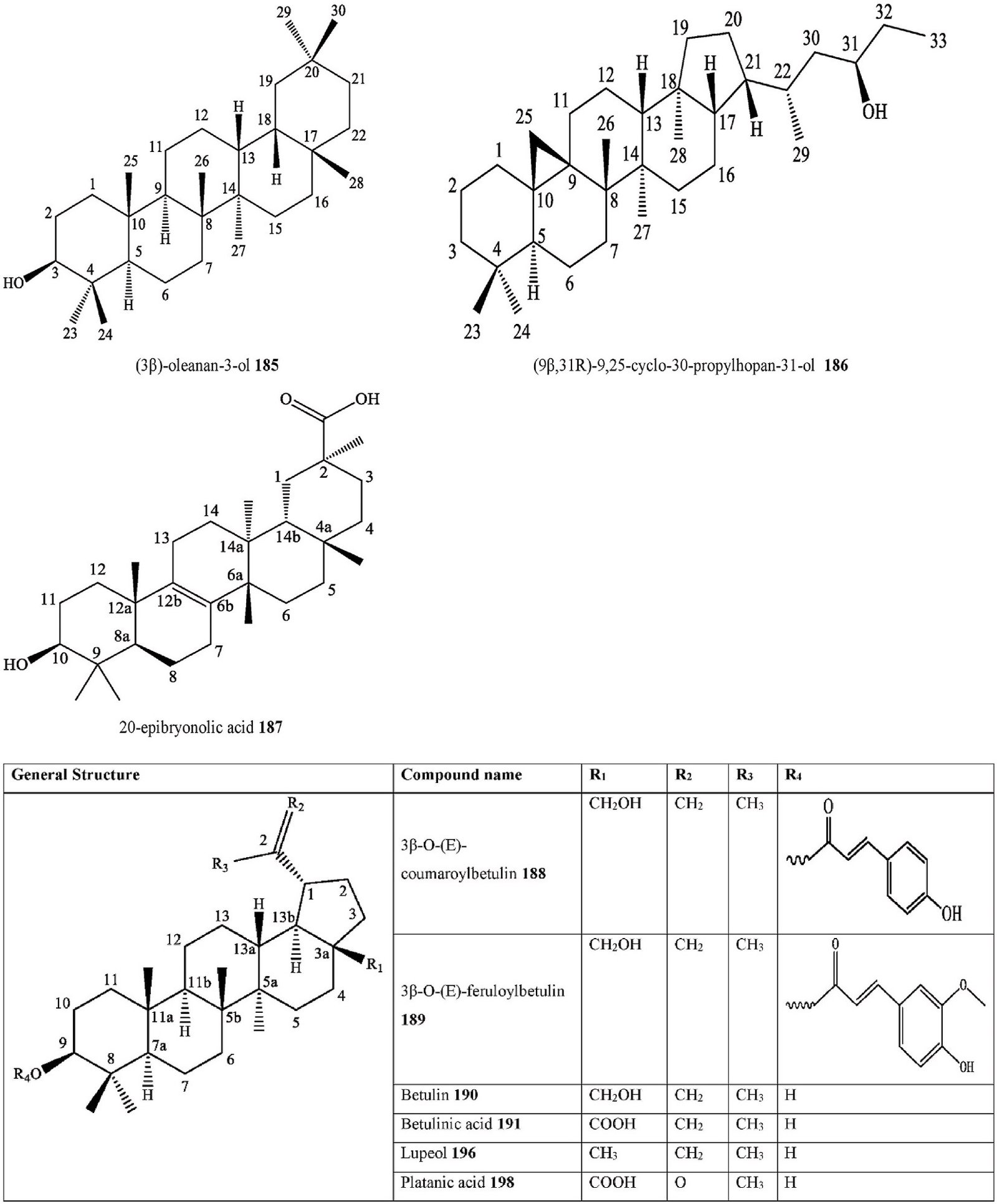
Fig. 6. (continued).
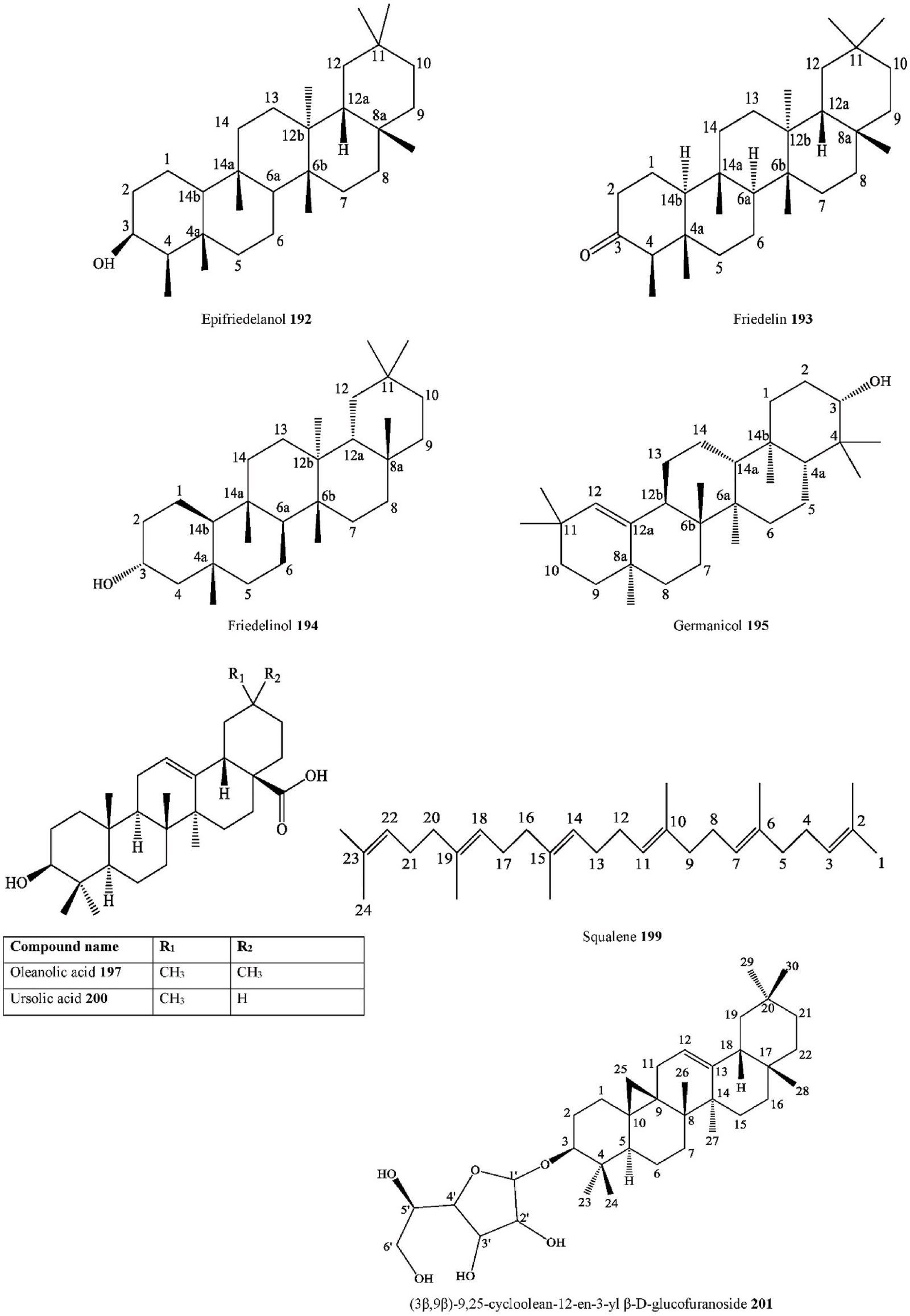
Fig. 6. (continued).
Three different extracts of C. tournefortii fruits displayed the growth inhibition of B. subtilis,Bacillus megaterium,S. aureus,E. coli,
P. aeruginosa,Listeria monocytogenes,K. pneumonia,P. vulgaris, and C. albicans [33]. Its water extract exhibited a narrow spectrum of activity and only showed inhibition against gram-positive bacteria (B. subtilis, B. megaterium, and S. aureus), while both ethanol and
Fig. 7. Miscellaneous compounds from the genus Celtis.
methanol extracts demonstrated broad-spectrum antibacterial activity, and methanol extract further showed antifungal activity against C. albicans [33]. In comparison to the growth inhibition activity of standard (10 mg/disc streptomycin sulfate and 30 mg/disc nystatin), methanol extract demonstrated superior antibacterial action against L. monocytogenes and B. subtilis [33]. Further studies are needed to identify the antimicrobial components of the methanol extract.
Among the plants of the Celtis genus, various extracts of different parts (leaves, seeds, and ripe fruits) of C. australis have notable antimicrobial activity against bacteria and fungus, even on resistance strains (Table 4). Leaf methanolic extract showed good anti- microbial potency against S. aureus and P. aeruginosa despite their resistance to Cefuroxime, Ampicillin, and Tetracycline. So, it is predictable that methanolic extract may have antibacterial components that show potency against resistant strains [30]. Another study
on ripe fruits of C. australis revealed that ethanol extract had potent antimicrobial effect against B. subtilis and P. aeruginosa (250 μg/ml and 125 μg/ml MICs, respectively) [31]. Furthermore, the ethanolic leaf (harvested at the end of October) extract has antifungal action against C. albicans, Candida parapsilosis (MIC 0.156 mg/mL), and R. mucilaginosa (MIC 0.313 mg/mL) [32], whereas the
= =
hydromethanol and ethyl acetate extracts of leaves and seeds have antifungal activity against C. albicans, Candida tropicalis, and
Aspergillus niger [138]. Among them, hydromethanol extract outperforms ethyl acetate extract in antifungal activity. In the case of
A. niger, hydromethanol extract of both seeds and leaves showed greater activity than the standard fluconazole. However, compared to nystatin, only leaves hydromethanol extract is as effective as nystatin [138]. Further hydromethanol extract of leaves study is needed to find out what the antifungal compound in them is. Along with anti-fungal action, ethyl acetate extract has remarkable anti-bacterial activity. The ethyl acetate extracts of the leaves and seeds are active against both gram-positive (Bacillus. spp,Bacillus cereus,Listeria ivanovii,and S. aureus) and gram-negative (C. freundii,E. coli, and S. sp) bacteria [138]. In particular, leaves ethyl acetate extract strongly reduced the growth of Citrobacter freundii and E. coli, while seeds ethyl acetate extract was more potent against Bacillus. spp,
L. ivanovii, and Staphylococcus spp. [138].
In the Celtis genus, various types of fatty acids are isolated from the species that may be involved in broad-spectrum antimicrobial activities. Recent biological research on fatty acids has found possible antibacterial mechanisms, such as inhibiting protein synthesis, DNA/RNA replication, cell wall, metabolic route, and quorum sensing (QS), as well as horizontal gene transfer (HGT), cytoplasmic membrane disruption, and efflux pumps, that may help reduce bacterial growth, even in resistant strains [146]. Compounds 154 and 156 are two familiar saturated plant-fatty acids also detected in these Celtis plants, both of which exert antibacterial action against gram-positive and gram-negative bacteria. Their nanostructure arrays successfully suppress the growth of P. aeruginosa and S. aureus [147], which are inhibited by the majority of various plant extracts of the Celtis genus (Tables 4 and 5).
They showed bactericidal action against vancomycin-resistant Enterococcus faecalis (VREF) and multidrug-resistant Staphylococcus epidermidis (MRSE) while encapsulated in liposome carriers [41]. Additionally, Parsons et al., stated that unsaturated fatty acid including compound 155 is noxious to metabolism because it is not good enough for phospholipid biosynthesis and accumulates in the cells of bacteria [148]. In this way, it affects the cell membrane and its functions, such as the proton gradient, and inhibits macro- molecular synthesis, which ultimately leads to energy loss [148]. Another phyto-fatty acid, compound 147, also alters the bacterial metabolic pathways of S. aureus [149] through its ability to alter gene expression in glycolytic and fermentative systems that are essential for energy production [146]. Furthermore, compounds 155 and 147 selectively inhibit bacterial enoyl-acyl carrier protein reductase (FabI), a key molecule in bacterial fatty acid generation [150]. The liposomal form of unsaturated fatty acid (compound 148)
exhibits minimum bacteriacidal concentration (MBC) against Helicobacter pylori at 200 μg/mL through increasing the permeability of
the outer membrane [151].
The presence of phenolic compounds in the Celtis genus may also be responsible for the enhancement of antibiotic activity even against resistant pathogens. Compound 297 displays antimicrobial activity on several microorganisms such as A. niger,Aspergillus fumigatus,Aspergillus flavus,Aspergillus ochraceus,Alternaria alternata,Botrytis cinerea,Candida. spp,Penicillium citrinum,Penicillium chrys- ogenum,Fusarium oxysporum, and Rhizopus oryzae through cell membrane disturbance [152]. The compound has bactericidal activity against H. pylori at low pH levels. However, the organism remained susceptible to the compound even after undergoing ten successive generations of growth at concentrations below their inhibitory levels, without developing any resistance [153]. Furthermore, this phytoconstituent also inhibits biofilm formation as well as breaks cell-to-cell communication in Methicillin-resistant Staphylococcus aureus (MRSA) at 0.04 % v/V concentration [42]. Compounds 71 and 65 inhibit vancomycin-resistant E. faecalis by binding to Beta-Ketoacyl-[acyl carrier protein]-synthase (KAS) III, which is required for bacterial fatty acid synthesis [154]. Other compounds, such as Genistein (aglycone of compound 123), compounds 112, 115, 114, 76, 122, and 305, exhibit activity against various mi-
croorganisms, even on resistant strains, at various concentrations [155–159]. Another mechanism of action, “inhibition of d-Alanine:
d-alanine ligase,” is shown by compounds 115 and 73 against H. pylori and E. coli [160]. Though compound 113 cannot affect bacterial growth, it can restrain the virulence of pathogenic bacterial strains, like S. aureus via Sortase A and B inhibitors [161].
Some terpenoids and their derivatives that have antimicrobial activity are also detected in Celtis genus plants (Table 3). Terpenes are more susceptible to gram-positive than gram-negative bacteria. Their lipophilic feature is mainly responsible for their antimi- crobial response [162]. Compound 190, a pentacyclic triterpenoid, has anti-staphylococcal activity against S. aureus. However, their individual actions are weaker than the common antibiotics. They produce a synergistic effect with the combination of beta-lactam and glycopeptide class antibiotics through cell wall inhibition. Among them, compound 191 and methicillin are the most effective com- binations [163]. Another familiar phyto-triterpenoid of Celtis species, compound 200, has broad-spectrum antibacterial activity. In the Langmuir monolayer technique, this phytoconstituent displayed a disorganizing effect on the applied model of the E. coli membrane [164].
Table 4
Biological activities of extracts of Celtis genus.
Activity Species Part
Extract
In vitro/ In vivo
Key findings Positive control/ Standard
Ref no.
Anti-diabetic activity C. philippensis Crude, Ethyl
acetate, Ethanol, and Aqueous extracts
Leaves In
vivo
Various solvent extract- treated groups of Wistar albino rats saw a considerable reduction in their peak blood glucose levels around day 14 of the experiment. However, the extract improved HDL levels relative to the glycemic group, indicating antilipidemic potential.
Glibenclamide [135]
Anti-diarrheal activity C. africana Aerial parts Organic fraction In
vivo
C. pallida Aerial parts Ethanol extract In
vivo
At a high dose, fractioned showed spasmolytic activity in rabbits through
the Ca++ antagonist
induced gut relaxation. Inhibited diarrheic defecation in BALB/c mice in a dose-dependent manner
Loperamide [136]
Loperamide [36]
Anti-inflammatory/ Analgesic activity
C. australis Barks, fruits, fatty acids (fruits)
Ethanol extracts of barks and fruits, fatty acids from ethyl acetate extracts
In vivo
On Swiss albino mice, 500 mg/kg extracts of barks and fruits and fatty acids showed a moderate analgesic effect against acetic acid-induced writhes.
On adult female Sprague- Dawley rats, crude extracts and fatty acids suppressed carrageenan- induced paw edema was significant at all concentrations (100 mg/
kg, 250 mg/kg, and 500 mg/kg) compared to the standard phenylbutazone.
Paracetamol and Phenylbutazone
[137]
C. choseniana Leaves Methanol extract In vivo, In vitro
C. pallida Aerial parts Ethanol extract In
vivo Anti-microbial activity C. africana Fruit Hexane extract In
vitro
C. africana Leaves Acetone extract In vitro
C. africana Leaves Hexane extract In vitro
In both in vivo and in vitro studies, it suppressed nitric oxide generation as well as mRNA expression of inducible nitric oxide synthase, tumor necrosis factor-alpha, and cyclooxygenase-2.
Decreased 30 % in ear inflammation of mice Against four types bacteria including E. coli,
P. mirabilis, S. aureus, and
B. cereus (MIC 32 mg/ml). Lowest MIC 4 mg/ml was recorded against
S. aureus.
Showed inhibition activity against
C. neoformans (MIC =
0.22 mg/ml)
Against P. aeruginosa, and
E. aerogenes (MIC 32 mg/ ml)
| C. africana | Stem | Ethyl acetate extract | In | Against K. pneumonia, | Streptomycin | [27] |
| vitro | P. aeruginosa, S. aureus, |
Prednisolone [68]
Indomethacin [36]
Streptomycin [27]
Amphotericin B [28]
Streptomycin [27]
Activity Species Part
Extract
In vitro/ In vivo
Key findings Positive control/ Standard
P. mirabilis, and E. coli
(MIC 32 mg/ml).
Ref no.
C. africana Stem bark Ethanol extract In
vitro
C. australis Leaves Ethanol extracts In vitro
Showed moderate anti- plasmodic activity against
P. falciparum 3D7 strain (IC50 = 29.05 μg/ml) More efficient against
C. albicans, C. parapsilosis (MICs = 0.156 mg/ml) than R. mucilaginosa(MIC
= 0.313 mg/ml).
| C. australis | Leaves and seeds | Hexane, and ethyl | In | Demonstrated better | Tetracyclin and | [138] |
| acetate extract | vitro | activity against Bacillus. | Penicillin G | |||
| sp, B. cereus, L. ivanovii, | ||||||
| C. freundii, and E. coli than | ||||||
| S. aureus. | ||||||
| C. freudii and E. coli were | ||||||
| significantly inhibited by | ||||||
| leaf ethyl acetate, | ||||||
| whereas B. sp, L. ivanovii, | ||||||
| and Salmonella sp were | ||||||
| C. australis | Leaves and seeds | Hydro-methanol and | In | more sensitive to seed
Extract of leaves showed |
Nistatine and | [138] |
| ethyl acetate extract | vitro | inhibition against A. niger | Fluconazole | |||
| and C. albicans | ||||||
| (hydromethanol) and | ||||||
| C. tropicalis (ethyl | ||||||
| acetate). Both leaves and | ||||||
| seeds ethyl acetate and | ||||||
| hydromethanol (leaves | ||||||
| and seeds) showed | ||||||
| inhibitory effects on | ||||||
| Candida albicans, while | ||||||
| fluconazole and nistatine | ||||||
| had no effect on them. | ||||||
| Hydromethanol > Ethyl | ||||||
| acetate. | ||||||
| Nystatin > | ||||||
| C. australis | Leaves | Water and methanol | In | Hydromethanol >
Against P. aeruginosa and |
Cephotaxime | [30] |
| extracts | vitro | S. aureus. Between the two | ||||
| extracts, methanol | ||||||
| showed the highest | ||||||
| antibacterial activity. | ||||||
| Activity was also recorded | ||||||
| against the resistance | ||||||
| strains of cefuroxime, | ||||||
| ampicillin, and | ||||||
| tetracycline. | ||||||
| Methanol > Water. | ||||||
| C. australis | Ripe fruits | Ethanol extract | In | Can be used in the case of
Activity against |
Ampicillin | [31] |
| vitro | P. aeruginosa and | |||||
| B. subtilis. MICs were 250 | ||||||
| μg/ml and 125 μg/ml, | ||||||
| C. laevigata | Plant materials | Dichloromethane | In | respectively
Showed better efficiency |
Rifampin | [35] |
| extract | vitro | against Mycobacterium | ||||
| tuberculosis (99 %) than | ||||||
| C. pallida | Aerial parts | Ethanol extract | In | Mycobacterium avium (39
Mild antimicrobial |
Cefotaxime | [36] |
| vitro | activity against B. subtillis, |
Artemisinin [29]
N/A [32]
ethyl acetate.
Fluconazole (A. Niger)
resistance
%)
Activity Species Part
Extract
In vitro/ In vivo
Key findings Positive control/ Standard
E. coli, P. aeruginosa, S. aureus, and C. albicans
Ref no.
C. tournefortii Fruits Ethanol extract In vitro
C. tournefortii Fruits Methanol extract In vitro
C. tournefortii Fruits Water extract In vitro
(MICs = 400 μg/ml).
Extract showed activity against L. monocytogenes,
E. coli, S. aureus,
P. aeruginosa,
K. pneumonia,
B. megaterium,
P. aeruginosa, B. subtilis bacteria, and C. albicans fungus.
Activity was recorded against P. vulgaris, B. megaterium, E. coli,
L. monocytogenes, P. aeruginosa, K. pneumonia,
B. subtilis, S. aureus bacteria and C. albicans fungus.
Better than standard (streptomycin and nystatin) against
L. Monocytogenes, and
B. Subtilis.
Activity was noted against
S. aureus, B. subtilis, and
B. megaterium.
Streptomycin sulfate (10 mg/ disc) and Nystatin (30 mg/disc)
Streptomycin sulfate (10 mg/ disc) and Nystatin (30 mg/disc)
Streptomycin sulfate (10 mg/ disc) and Nystatin (30 mg/disc)
[33]
[33]
[33]
C. tournefortii Leaves Aqueous extract (Silver Nanoparticles)
In vitro
Silver nanoparticles at doses of 0.06–0.13 μg/mL and 0.50–1.00 μg/mL
showed effective inhibitory action against gram-positive bacteria
S. aureus and B. subtilis, while gram-negative bacteria E. coli, and
P. aeruginosa. Silver nanoparticles were also effective against
C. albicans growth at 0.03 g/mL, a significantly lower dosage than antibiotics.
[34]
Antiulcerogenic activity
C. iguanaea Ethanolextract Leaves In vivo
C. iguanaea Hexane extract Leaves In vivo
The activity was shown to protect from indomethacin, ethanol, and pyloric ligation- induced gastric ulcers in male Swiss mice. The hexane fraction of this extract reduced indomethacin-induced ulcers by suppressing the release of gastric acid, increasing pH, and decreasing acidity without interrupting intestinal motility through the anticholinergic mechanism.
The activity of this species reduced indomethacin and pyloric ligation-
Ranitidine [139]
Ranitidine [140]
Activity Species Part
Extract
In vitro/ In vivo
Key findings Positive control/ Standard
induced gastric ulceration and lesion index in the experimental models. It blocked the histamine and cholinergic receptors that hindered the cell molecular events of gastric secretion as well as
suppressed the total H+
excretion.
Ref no.
Cytotoxic /Anticancer activity/ Antiproliferative activity/Anti- tumor activity
C. aetnensis Twigs Chloroform extract In vitro
Extract reduced human colon cancer cell line (Caco2) cells by apoptosis at the low dose (5 μM) and necrosis at high dose (250 μg/ml). This extract increased ROS levels,
decreased RSH levels, and increased heme oxygenase (HO-1) expression.
Untreated control group
[38]
C. africana Aerial parts Ethyl acetate extract In
vitro
Showed the highest
cytotoxicity (EC50 = 8.3
μg/ml) among the other
extracts such as petroleum-ether, chloroform, and n- butanol against mouse lymphoma cells L5178Y, while positive control Kahalalide F exhibited an
EC50 of 6.3 μg/ml.
Kahalalide F [37]
C. eriocarpa Leaves Methanolic extract, n- Hexane fraction, Chloroform fraction, Ethyl acetate fraction, and Aqueous fraction
In vitro
The highest cytotoxin LC50 was noted from ethyl acetate fraction against Brine shrimp larva at
243.61 μg/ml, while
positive control potassium dichromate
revealed LC50 at 7.04 μg/
ml. Among them, the LC50 value ranged from 243.61
μg/ml to 1015 μg/ml. The
n-hexane fraction produced the lowest activity.
Ethyl acetate fractions >
methanol extracts > chloroform fractions > Aqueous fractions > n- Hexane.
Potassium Dichromate
[118]
C. eriocarpa Leaves Methanolic extract, n- Hexane fraction, Chloroform fraction, Ethyl-acetate fraction, and Aqueous fraction
(In vivo/ In vitro)
Compared with camptothecin (positive control), activity was shown against Agrobacterium tumefaciens induced tumors on potato discs, but the result was insignificant.
Camptothecin showed an IC50 value of 3.67 μg/ml, while leaf extracts’ IC50 values ranged from 372 μg/ml to 1057 μg/ml.
Ethyl acetate fraction >
Methanol extract >
Camptothecin [118]
Activity Species Part
Extract
In vitro/ In vivo
Key findings Positive control/ Standard
Chloroform fraction > Aqueous fraction > Hexane fraction
Ref no.
C.iguanaea Leaves Dichloromethane, and Hexane extract
In vitro
Dichloromethane showed activity against human ovarian (OVCAR-3), lung (NCI–H460), and
glioblastoma (U-251) tumour cells, with GI50
values of 28.46 μg/ml,
32.31 μg/ml, and 37.99
μg/ml, respectively.
On the other hand, hexane extract showed activity against human glioblastoma (U-251), ovarian (OVCAR-3), and colon (HT-29) tumour cells, with GI50 values of
6.40 mg/ml, 3.99 mg/ml, and 3.16 mg/ml, respectively.
Hexane extract >
Dichloromethane extract
Doxorubicin
[108]
C. tournefortii Fruits Ethanol extracts In vitro
C. tournefortii Fruits Methanol extracts In vitro
C. tournefortii Fruits Water extracts In vitro
Ethanol extract demonstrated better activity than water and methanol extracts against PC-3.
Methanol extract exhibited better activity than water and ethanol extracts against A2780. Water extract showed better activity than ethanol and methanol extracts against MCF-7, HCT-116.
Cell were treated with DMSO (Solvent-control group)
Cell were treated with DMSO (Solvent-control group)
Cell were treated with DMSO (Solvent-control group)
[33]
[33]
[33]
C. tourneforti Leaves Aqueous extract (Silver-nanoparticle)
In vitro
Silver nanoparticles of leaves extract showed effective on CaCo-2 cell line. Morever, low activity was detected against healthy cell line HDF.
[34]
Healing wounded C. australis Seeds Ethyl acetate extract In
vivo
| Hepatoprotective | C. tournefortii | Fruits | Aqueous, 25 % | In | Activity was shown to | N/A | [142] |
| ethanol, and 75 % | vivo | protect against Cu- | |||||
| ethanol | induced hepatic cell | ||||||
| damage in Wistar Albino | |||||||
| rats. Fruit extracts | |||||||
| significantly emaciated | |||||||
| the degenerative and | |||||||
| necrotic destruction of the | |||||||
| Cu-induced hepatic | |||||||
| damage in the rats. It may | |||||||
| increase the antioxidant | |||||||
| activity that assuages the | |||||||
| destruction of the Cu- | |||||||
| C. tournefortii | Leaves | Aqueous, ethanol- | In | Activity was shown to | N/A | [143] | |
| aqueous (1:3 v/v), and | vivo | protect against CCl4– | |||||
| ethanol-aqueous (3:1 | induced hepatic cells | ||||||
| v/v) | damage in Wistar albino | ||||||
In Sprague-Dawley rats, the wound healing rate was as same as the standard ointment rates.
Mad´ecassol® [141]
induced toxicity.
Activity Species Part
Extract
In vitro/ In vivo
Key findings Positive control/ Standard
rats. Results revealed that the leaf extract tremendously lessened the CCl4-induced degenerative and necrotic
destruction of the rat’s
hepatic tissue by enhancing the antioxidant activity. It has the potential to be used as a hepatoprotective agent.
Ref no.
Laxative (prokinetic) C. africana Aerial parts Aqueous fraction In
vivo
Rabbits demonstrated dose-dependent spasmogenic action at low
dosages of 0.03–3 mg/ml,
which contracted the
rabbit’s jejunum. It displayed atropine-
sensitive prokinetic and laxative actions.
Carbachol [136]
Rumen fermentation C. pallida Leaves Hydroalcoholic extract N/A Significantly
improvement of rumen fermentation at doses 1.2–1.8 ml/g dry matter
of diets
N/A [144]
After the aforementioned, it can be concluded that the various mechanisms of antimicrobial activity of Celtis species depend on the plant compounds as well as the types of extract solvent (polar and non-polar). Furthermore, Celtis may show some hope for antimi- crobial resistance disaster, because of some isolated compound of Celtis showed positive effect on the VREF, MRSE, and MRSA. However, the majority of the published articles are based on in vitro tests, which may not assure the same results in animal models or clinical conditions. With the increase of antibiotic-resistant pathogenic bacteria, there is an urgent need to find novel antimicrobial drugs, while phytoconstituents from plants such as Celtis could be promising alternatives.
Cancer is among the ailments that kill large numbers of people every year throughout the world. A study shows that in southern Thailand raw seed consumption has remarkable healing properties in the occurrence of esophageal cancer [165]. Among three different extracts of C. tournefortii fruits, the water extract showed better activity against human breast cancer (MCF-7) and human colon cancer (HCT-116) than the ethanol and methanol extracts. However, ethanol extract showed superiority against human prostrate cancer (PC-3), while methanol extract was more efficient against human ovarian cancer (A2780) cell lines [33]. A new glucos- phingolipid (compound 235), isolated from C. africana, displayed potent cytotoxicity against mouse lymphoma cells L5178Y, nearly the same as the positive control Kahalalide F and better than other extracts such as ethyl acetate, petroleum-ether, chloroform, and n-butanol extract [37] (Table 5). Methanolic extract and its various fractions of C. eriocarpa leaves exhibited cytotoxicity against Brine shrimp larvae, while the ethyl acetate fraction showed more efficiency than the other fractions (n-Hexane, chloroform, and aqueous) and the methanolic extract [118]. Another chloroform extract of C. aetnensis twigs induced apoptosis in a Human Colon Cancer (Caco2) cell lines at a low dose, and necrosis at a high dose through the increase of reactive oxygen species (ROS) levels, heme oxygenase (HO-1) expression, and decreasing reactive thiol group (RSH) levels [38].
Some familiar plant bioactive compounds are also identified from investigated Celtis species to have tremendous anticancer ac- tivity. Compound 147 is such a bioactive compound and one of the frequently occurring fatty acids in Celtis species (Table 3), that in high doses decreases the proliferation of Caco-2 cell line [166], with a protective effect against cancer growth [167]. Another fatty acid of Celtis species, compound 154, demonstrated selective cytotoxicity by promoting apoptosis in the human leukemic (MOLT-4) cell line. Compound 154 exerts an anticancer effect in mice by targeting tumor cell DNA topoisomerase I. Surprisingly, it does not affect DNA topoisomerase II, indicating that compound 154 can be used as an anti-cancer medicine [168]. Furthermore, conjugation of N-acylhydrazones, with compounds 149, 153, and 147 displays activity against human breast cancer (MCF-7), leukemia (HL-60),
cervix (KB–V1/Vbl), and melanoma (518A2) carcinomas, while conjugation with compounds 149 is three times better than Doxo- rubicin [169]. A familiar plant’s flavone glycoside, compound 105, isolated from four distinct Celtis species (C. africana,C. australis,
C. occidentalis, and C. iguanaea) suppresses cell growth, invasion, and migration. In Adeno-carcinomic human alveolar basal epithelial (A549) cell lines, it reduces Cyclooxygenase-2 (COX-2) messenger RNA (mRNA) expression by upregulating MicroRNA 26b (miR-26b) and MicroRNA 146a (mir-146a) [170]. Furthermore, the compound 105 and celecoxib combination demonstrate a synergistic impact
Table 5
Biological activites of the phytoconstituents of Celtis genus.
Activity name Comp. Number Plants In
vitro/ In vivo
Key findings Positive control/
Standard
Ref. no
Anticholinergic activity
Compound 8 C. sinensis In vivo
Exhibited a dose-dependent acetylcholinesterase inhibitory effect in a dose- dependent response at male ICR mice
Berberine [104]
Compound 8,
compound 10,
compound 6
C. africana In vitro Three of them showed moderate
acetylcholinesterase inhibitors effects. Compound 10 > compound 6 > compound 8
Galanthamine [102]
Anti-inflammatory/ analgesic activity
Compound 125 C. adolphi- friderici
In vitro Compound 125 (IC50 = 16.3 μM) showed high
potent anti-inflammatory activity, even better than standard Baicalein.
Baicalein [103]
Compound 131 C. tessmannii In vitro Activity against lipoxygenase was more than Baicalein [97]
Compound 101 C. sinensis In vivo
the standard (Baicalein). (IC50 = 12.9 μM) In vivo, compound 101 decreased inflammatory molecules (IFN-α, TNF-α, IL-2,
and IL-17A) in the lymphatic system, inhibited cytokine release into the serum, and increased apoptosis-related protein production in ginkgo acid-induced contact dermatitis in ICR mice. Additionally, in vitro, Con A-activated T cells showed death and decreased inflammatory cytokines. This chemical blocked MAPK and STAT signaling and phosphorylated SHP2.
Dexamethasone [124]
Compound 8,
compound 10,
compound 6
C. africana In vivo In rats’ carrageenan induced paw edema,
compound 8 exhibited remarkable action,
whereas compounds 3 and 4 exhibited only mild action. compound 8 > compound 10 > compound 6
Diclofenac sodium
[102]
Anti-microbial activity
Compound 138 C. australis In vitro Activity was shown against gram positive
bacteria including B. sp, B. cereus, L. ivanovii, and S. aureus. (MICs = 25–100 μg/ml). Most active against B. cereus (MICs = 25 μg/ml)
Demonstrated activity against gram negative bacteria such as C. freundii, E. coli, and S. sp.
Tretracycline [127]
Compound 191 C. tessmannii In vitro
(MICs = 25–100 μg/ml)
Showed potency anti-plasmodium activity against various chloroquine-sensitive and
resistant P. falciparum strains. (IC50 = 2.38–1.7
μg/ml)
N/A [97]
Compound 301 C. australis In vitro Against gram positive bacteria such as B. sp,
B. cereus, L. ivanovii, and S. aureus. (MICs =
100–200 μg/ml)
Against gram negative bacteria including
Tretracycline [127]
Compound 303 C. australis In vitro
E. coli and S. sp. (MICs = 200 μg/ml)
Showed activity against gram positive bacteria
such as B. sp, B. cereus, L. ivanovii, and S. aureus. (MICs = 50–200 μg/ml)
Activity was demonstrated against gram negative bacteria such as C. freundii, E. coli, and
Tretracycline [127]
Cytotoxicity/Anti- cancer activity/ Anti- proliferative activity/Anti- tumor activity
Compound 200 C. philippinensis In vitro
S. sp. (MICs = 100–200 μg/ml)
Activity showed better against oral epidermoid than other such as against human lung, colon, oral epidermoid, and hormone-dependent prostate cancer.
oral epidermoid > hormone-dependent
prostate > colon > lung
Paclitaxel and Camptothecin
[115]
Compound 53 C. philippinensis In vitro Activity showed better against oral epidermoid
than other such as against human lung, colon, oral epidermoid, and hormone-dependent prostate cancer.
oral epidermoid > hormone-dependent
prostate > colon > lung
Compound 54 C. philippinensis In vitro Activity showed better against oral epidermoid
than others such as human lung, colon, oral epidermoid, and hormone-dependent prostate cancer.
oral epidermoid > hormone-dependent
prostate > lung > colon
Paclitaxel and Camptothecin
Paclitaxel and Camptothecin
[115]
[115]
(continued on next page)
Table 5 (continued )
| Activity name Comp. Number | Plants | In vitro/ | Key findings | Positive control/ | Ref. no |
| Compound 288 | C. africana | In vitro | Demonstrated better cytotoxicity (EC50 = 7.8 | Kahalalide F | [37] |
In vivo
Standard
μg/ml) than other extracts, such as ethyl
acetate, petroleum-ether, chloroform, and n- butanol extract, against mouse lymphoma cells L5178Y, as well as near to the standard Kahalalide F.
Compound 302 C. iguanaea In vitro Activity was shown against human liver,
breast, colon, and lung tumor cell lines through cell cycle arrest and apoptosis.
Doxorubicin
[108]
Compound 58,
compound 60,
compound 59
Compound 63,
compound 64
C. tetrandra In vitro Compounds demonstrated remarkable activity
in overcoming TRAIL (Tumor necrosis factor (TNF)-related apoptosis-inducing ligand) resistance in AGS (human gastric adenocarcinoma) cells. It can be used to treat the TRAIL resistance AGS cell.
C. tetrandra In vitro These two flavanol dimers showed low potency
to overcome TRAIL resistance in AGS cells.
Luteolin [116]
Luteolin [116]
Urease inhibitory Compound 131 C. tessmannii In vitro Compound 53 was reported as having the most
potent anti-urease activity even more than the standard thiourea.
Thiourea [97]
Compound 193 C. adolphi- friderici
Compound 77 showed the very high potent anti-urease activity even more than thiourea (standard).
Thiourea [103]
Compound 301 C. zenkeri In vitro It was more potent inhibitor against the Jack
bean urease (IC50 = 20.3 μM), than the
Thiourea [90]
Compound 304 C. zenkeri In vitro
standard (thiourea- IC50 = 21.5 μM)
In comparison to the standard (thiourea- IC50
= 21.5 μM), it was high moderate inhibitor of
Thiourea [90]
Compound 108,
compound 100,
compound 99,
compound 105,
C. africana In vitro
the Jack bean urease (IC50 = 27.6 μM). Compound 108, compound 105, compound 99 and compound 100 showed potent urease inhibitory activity.
Compound 105 > compound 108 > compound 99 > compounds 100
Thiourea [123]
*Compound number indicates the compound’s serial number of Table 3.
on cell invasion and migration in A549 cell lines through the inhibition of COX-2, inducible nitric oxide synthase (iNOS), and B-Cell Leukemia 2 expression with the activation of the apoptosis-inducing gene “Cytochrome P450 Family 1 Subfamily A Member 1” [170]. The results show that both compound 105 and its combination could be a potentially effective medicine that kills cells by causing inflammation.
Cytotoxic terpenoids are also detected from Celtis genus. For example, compound 199, which is separated from fruits of C.africana [27], can reduce a significant portion of rats’ aberrant crypt foci (ACF) in the colon. The possible mechanism is that it may be able to stop “3-Hydroxy-3-Methyl-Glutaryl-Coenzyme A Reductase” or bile acids that lead to colonic tumors or ACF [171]. Compounds 58 and 60, two flavanol epimers of the bark of C. tretranda, contribute to human gastric adenocarcinoma (AGS) cells regain from Tumor
Necrosis Factor Related Apoptosis-Inducing Ligand (TRAIL) resistance-overcoming properties much more than their dimers, com- pounds 63 and 64 [116].
Because of their ability to block the expression of numerous tumor-and angiogenesis-associated genes, phytoconstituents may also enhance apoptotic signaling channels by reducing activating caspases, as demonstrated by analogous molecules from other plant genera [172], along with significant downregulation of DNA synthesis. Additional investigation is needed to put emphasis on the probable pharmacological mechanisms that are involved in anticancer activity. Also, the results of the experiments need to be backed up by a lot of research on human carcinoma cell lines.
Almost every clinical manifestation is accompanied by a proinflammatory response. As a result, the anti-inflammatory properties of Celtis plant materials may be useful. Various kinds of inflammation have been used to test the anti-inflammatory activities of C. australis barks and fruits [137], C. pallida aerial parts [36], as well as C. choseniana leaf extracts [68].
The anti-inflammatory properties of ethanolic extracts of barks and fruits of C. australis were reported via their remarkable
reduction of carrageenan-induced paw edema. The same study also revealed the analgesic effects of C. australis by inhibition of acetic acid-induced writhes in Swiss albino mice [137]. However, the extracts’ outcomes against inflammation were better than the fatty acid experiment [137]. Leaves of C. choseniana decrease nitric oxide generation as well as mRNA expression of iNOS, COX-2, and tumor necrosis factor-alpha (TNF-α) [68]. Further investigation revealed that this extract contained anti-inflammatory flavonoids such as
compounds 112, 115, and 76 [68].
Compound 25, an isolated phytoconstituent from C. africana fruits, leads to anti-inflammatory effect by suppressing iNOS and COX- 2 [173], whereas compound 131 of C. tessmannii acid shows activity by inhibiting lipoxygenase [97] (Table 5). Compound 148 also
reduces lipoxygenase induced interleukin-1 (IL), IL-6, and TNF-α [97]. Additionally, TNF-α production is also inhibited by C. sinensis
lignan glycoside [101].
From the aforementioned, it is apparent that Celtis genus bioactive molecules decrease inflammatory components through the interrupting cyclooxygenase, and lipoxygenase pathway, which may lead to reduce the generation of inflammatory mediators such as IL and TNF-α.
The antidiarrheal effect of the Celtis genus has been investigated through many animals’ model, such as rabbits and BALB/c mice. The chloroform fraction of ethanol: water (8:2) extract of C. africana aerial parts reduced the frequency of stooling in rabbits at a high dose [136]. An ethanol extract of C. pallida aerial parts exhibited dose-dependent antidiarrheal activity via diarrheic defecation in
BALB/c mice [36]. Conversely, the aqueous fraction of ethanol: water (8:2) extract of C. africana aerial parts demonstrated
atropine-sensitive prokinetic activity at lower dose by contracting rabbits’ jejunum [136]. Medicinal plants are generally known to have antidiarrheal properties through stimulating the intestinal K+ channels and activating Na+/K+- ATPase activity, as well as reducing intracellular Ca++ concentration, facilitating gastrointestinal smooth muscle relaxation as well as reducing diarrhea
[174–176]. Further studies are necessary to isolate the potential anti-diarrheal compounds from Celtis species extracts, which may lead to get a new gut relaxation agent.
Acetylcholinesterase is responsible for the cessation of signal transduction of several cholinergic systems in the central and pe- ripheral nervous systems by efficiently breaking down the neurotransmitter acetylcholine [177]. From the Celtis genus, different hydroxycinnamic acid derivative amide compounds, 8, 10, and 6, were detected in the ethanol-water extract of C. africana aerial parts, which showed a weak to moderate acetylcholinesterase inhibition activity [102]. Compound 8 is also isolated from twigs of C. sinensis [104]. As per their structure, compound 8 has a hydroxy group at the 4th position, while compound 10 has an extra methoxy group at the 3rd position that may be accountable for its better activity. However, the most active constituent among them, compound 6, has two additional hydroxy groups at the 3rd and 4th positions, which may be responsible for the strongest activity [102]. As per our knowledge, despite their structure-activity-relationship, the exact mechanism of action against acetylcholinesterase is still obscure. Additional investigation is needed to learn about their mechanism of action, which may lead to the invention of a novel acetylcho- linesterase inhibitory molecule.
The nickel-containing enzyme, urease, plays a vital role in the breakdown of urea to generate ammonia and CO2 [178]. The urease activity of H. pylori plays a crucial role in the etiology of gastric and peptic ulcers [179]. So, plant-urease inhibitors are potent compounds that can be used as anti-ulcer medications. The urease inhibitory effect of Celtis plants was revealed in several isolated compounds of three species including C. adolphi-friderici, C. tessmanii, and C. africana (Table 4). A triterpene, compound 193, detected
from the roots of C. adolphi-friderici exhibited more potent anti-urease activity (50 % inhibitory concentration (IC50) = 15.36 μM) than other isolated compounds from this species, even more, effective than standard thiourea (IC50 = 21.6 μM) [103]. Compound 131, another phytoconstituent, that was isolated from C. tessmanii, also had more efficacy anti-urease activity (IC50 12.9 μM) than thiourea [97] (Table 5). Four constituents, including compound 108, compound 105, 99, and 100 of C. africana demonstrated potent
=
anti-urease activity, while the other three compounds 94, 95, and 109 were not as efficacious as the previous four constituents [123]. As per their structure, the presence of a sugar moiety might reduce their potential anti-urease activity [37].
Isolated compounds of Celtis species have tremendous potential as urease inhibitory constituents. Despite their remarkable activity against the urease enzyme, their precise mechanism of action remains unknown. Moreover, all investigation has been done under an in vitro test. A clinical trial is necessary to evaluate their in vivo potency, which may lead to the establishment of a new potent anti-urease medication.
Aside from the previously mentioned activities, the isolated phytoconstituents and extracts have several protective functions. For example, compounds 148 and 199 have neuro- and hepatoprotective effects, respectively [180,181]. Ethyl acetate extract of
C. australis seeds had remarkable wound healing efficacy comparable to that of standard ointment [141]. Furthermore, detected flavonoids such as compounds 106, 67, and 69 have cardioprotective properties due to their immunosuppressive and antioxidant properties [182–184]. More research is needed to investigate the various processes involved in the aforementioned positive benefits.
Antioxidant properties
Besides performing several biological functions, extracts and compounds of Celtis plants exhibit remarkable antioxidant activity (Tables 6 and 7). Their abilities to quench singlet oxygen and react with a variety of radical species may help to reduce oxidative stress in humans. So, it may help protect against diseases like heart disease and cancer [185]. Leaves and fruits extracts of Celtis plants display antioxidant activities in various tests (Table 6).
In a 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) scavenging test, methanolic extract of C. africana stems showed better antioxidant
activity than leaves [186]. Another hackberry (C. australis) showed different antioxidant properties at DPPH, and ferric reducing antioxidant power assay (FRAP) based on their different genotpes. The varying antioxidant potential estimated using FRAP and DPPH
tests was 44.35–117.87 mg Fe2+/100 g and 14.12–88.24 %, respectively [110]. Furthermore, Synergism activity is also noticed in the
Celtis genus. For instance, the n-butanol fraction of C. africana aerial parts exhibited an IC50 value of 40.5 μM, which is better than the other isolated compounds of this fraction >42 μM [123]. Water extract of C. tournefortii fruit showed more efficacious in hydroxyl radical (OH—) scavenging test than the standard butylated hydroxytoluene (BHT) [33]. Also, hydroalcoholic extracts of C. iguanaea
leaves showed antioxidant activity in a rat model by lowering the levels of thiobarbituric acid reactive substances (TBARS) (byproducts of lipid peroxidation) in the plasma and raising the levels of nonprotein thiols (CI-600) [121].
Almost all investigated Celtis plants contain a variety of flavonoids, including flavanol, flavone, isoflavone, flavonol, and flava- nonol, which have been known for their antioxidant activity due to their ability to act as hydrogen donors as well as reducing agents [188]. Flavonoids suppress the enzymes that produce superoxide anions, such as protein Kinase-C and xanthine oxidase. They can also inhibit microsomal monooxygenase, cyclooxygenase, mitochondrial succinoxidase, lipoxygenase, glutathione S-transferase, and nicotinamide adenine dinucleotide oxidase, all of which are involved in the generation of ROS. Furthermore, flavonoids effectively chelate trace metals that are needed in oxygen metabolism [189]. Three flavanols, compounds 59, 61, and 62, are reported from the Celtis plants (Table 3), where compounds 59 and 62 demonstrated similar antioxidant properties in the DPPH scavenging test (nearly
Table 6
Antioxidant properties of various extractives of Celtis genus.
Species Part
Extract
In vitro/ In vivo
Key findings Positive
control/ Standard
Ref no.
C. africana Aerial parts
Ethanol extract (n-butanol fraction)
In vitro Showed anti-oxidant activity while IC50 value was 40.5 μM (DPPH scavenging test). That was better than the other isolated compounds > 42 μM.
Maybe it’s a synergism effect of isolated compounds
from this extract
BHA [123]
C. africana Leaves and stems
Methanol extract In vitro At 0.1 mg/ml concentration, stem showed better
activity than leaves (DPPH testing). It is less effective than the standard ascorbic acid and butylated hydroxytoluene (BHT).
Stems > Leaves
Ascorbic acid, BHT
[186]
C. australis Leaves Hydroalcoholic extract In vitro Comparison to the Ascorbic acid (IC50 = 14.3 μg/ml), it
showed lower activity (IC50 = 80.5 μg/ml) in DPPH
scavenging test.
Ascorbic acid [187]
C. eriocarpa Leaves Methanol extracts and sub
fraction
In vitro Ethyl acetate fraction showed greater activity than others including hexane, chloroform, aqueous fractions and methanol extracts (DPPH testing).
Ethyl acetate fractions (EC50 = 324.81 μg/ml) >
methanol extracts (EC50 = 593.68 μg/ml) > chloroform fractions (EC50 = 1058.18 μg/ml) > aqueous fractions (EC50 = 1155.0 μg/ml) > hexane fractions (EC50 = 2981.03 μg/ml).
Ascorbic acid [118]
C. iguanaea Leaves Hydroalcoholic extract In vivo Activity was observed in rats’ plasma by the decrease of
TBARS (Thio-barbituric acid reactive substances) and an increase in nonprotein thiol levels (CI-600).
Simvastatin [121]
C. pallida Leaves Methanol, methanol:
water (80:20), and acetone extract
In vitro In the DPPH scavenging test, acetone extract showed better activity than other two including methanol, and methanol: water (80:20).
acetone > methanol > methanol: water (80:20)
BHA,
α-tocopherol
[117]
C. tournefortii Fruits Water, ethanol and
methanol extracts
In vitro In the DPPH radical scavenging testing, activity was lower than standard BHT. However, in the OH— scavenging testing fruits water extract (84.12 %) exhibited higher antioxidant activity than BHT (75.77
%).
BHT [33]
C. zenkeri Leaves and stem barks
Essential oils In vitro At 250 μg/ml, leaves showed tremendous antioxidant
activity compared to standard ascorbic acid and BHA, while being higher than α-tocopherol (DPPH testing). Stem barks showed the same potent activity as
standards (ascorbic acid and BHA) at any concentration, more than α-tocopherol.
Ascorbic acid and BHA
[99]
Table 7
Antioxidant properties of the new phytoconstituents of Celtis genus.
Standard
| Comp. Number | Plants | Invitro/ In vivo | Key findings | Positive control/ | Ref. no |
| Compound 138 | C. australis | In vitro | In the DPPH scavenging test, the IC50 value was 8.2 μg/ | BHT | [127] |
ml, while the BHT IC50 was 12.0 μg/ml.
Compound 131 C. tessmannii In vitro In the DPPH radical scavenging test, compound 53 was
better antioxidants than the standard.
BHA [97]
Compound 81 C. australia &
C. occidentalis
In vitro Showed greater activity against superoxide radical (induced by xanthine oxidase) as well as DPPH radical scavenging testing than the standard.
α-tocopherol, and BHT
[52]
Compound 8, C. africana In vitro In the DPPH scavenging test, compound 6 is more active BHA [102]
Compound 10,
Compound 6
Compound 6, compound 10,
compound 9, compound 7,
compound 4, compound 8
than others two compounds. Compound 6 and compound 10, were better than standard BHA (26.3 μm and 33.2 μm, respectively).
Compound 6 > compound 10 > compound 8
C. occidentalis In vitro In the DPPH scavenging testing, compound 6 and
10showed remarkable antioxidants activity. Compound 6 > compound 10 > compound 9 >
compound 7 > compound 4 > compound 8
Caffeic acid (IC50
= 4.6 ± 0.3)
[100]
Compound 138, compound
141, compound 125
C. adolphi-friderici In vitro In the DPPH scavenging test, compound 125 showed
tremendous activity compound 141, and compound 138. While all compounds demonstrated good antioxidant as well as better than the standard BHA
Compound 125 > compound 141 > compound 138 >
standard BHA.
BHA [103]
Note: Compound number indicates the serial number of the compounds displayed in Table 3.
80 % effective), while compound 61 was greater than them (85 % effective). But low-density lipoprotein (LDL) oxidation and FRAPassays showed that compounds 59 and 61 were equally effective (Table 7) [190].
Compound 104, a flavonol glycoside, is isolated from four different Celtis species (C. australis, C. tournefortii, C. occidentalis, and
C. iguanaea) (Table 7), and exhibits a variety of protective actions via an antioxidant mechanism. For example, it acts as a ROS scavenger where it increases glutathione production as well as improves cellular oxidative defense mechanisms by upregulating numerous antioxidant enzymes, including catalase and superoxide dismutase [191]. Additionally, compound 104 inhibits xanthine oxidase, which is also responsible for the production of ROS [191]. It also displays several neuroprotective activities in various in vitro and in vivo studies, through the reduction of ROS, lipid peroxidation, and iNOS [191]. Another apigenin flavone glycoside of the Celtis
genus, compound 114 and its various derivatives (Table 3) also have remarkable antioxidant as well as protective activity where it reduces the growth of lipid peroxidation, nitrite levels, and neuronal degeneration. It recovers the acetylcholinesterase–monoamine enzyme to its normal range and reduces the expression of mRNA of the metabotropic glutamate receptor 1, N-methyl– D-aspartate-receptor, and metabotropic glutamate 5 [192]. Another study of compound 114 (15 mg/kg, i. v.) showed that it improved
the neurological dysfunction in cerebral ischemia/reperfusion by boosting extracellular signal-regulated kinase ½ and BCL-2 protein levels in the cortex and hippocampus while diminishing BCL-2 associated X protein expression, jun N-terminal kinases, and p38 phosphorylation [193].
Because of having conjugated double bonds, terpenoid compounds have the ability to quench singlet oxygen, hydrogen or electron transfer. Such as isolated terpenoids of C. australis fruits, compound 83 showed greater antioxidant activity than compound 82 due to the presence of additional double bonds in compound 83 [185]. Along with oxygen radicals, terpenoids also scavenge several radicals. Compound 82, for instance, scavenges sulfur radicals, whereas compound 84 scavenges sulfonyl, nitrogen, and glutathione radicals
[185]. Tocopherols (compounds 85–88) can move hydrogen atoms from one molecule to another, which changes lipids and peroxyl radicals into more stable substances [194].
Other uses
Along with traditional and pharmacological uses, Celtis plants are also known for their decorative [195], furniture, millwork, and box manufacturing purposes [195,196]. Apart from decorative use, C. africana wood is used for flooring, construction, fuel, and charcoal manufacturing [27]. C. occidentalis roots are used to make dye [197], while the bark of C. australis is used to make yellow dye [198]. Furthermore, the woods of C. australis are used as fuel [199], agriculture equipment, and handle manufacturing [88]. Malleable thin shoots are used as walking sticks [197]. The timber of C. tetranda is strong, and durable and is used for manufacturing handle ores as well as fuel [199]. Roots of C. pallida are much strong to use in erosion problems [200]. However, the timber of C. laevigata and
C. pallida is not good enough. They are used only for fencing and fuel [199,201,202].
Limitations
The review could be more flawless. The specified phytoconstituents of the genus or delve into their intricate mechanisms of action were not thoroughly examined primarily due to insufficient evidence regarding precise mechanistic details. In addition, the review
lacks the crucial ethnopharmacological information. The ethnopharmacology section would benefit from enhancements by incor- porating specific criteria for interpreting ethnobotanical data. This could involve utilizing qualitative citation metrics like Relative Frequency Citation (RFC), Fidelity Level (FL), Relative Importance (RI), and Frequency Index (FI). However, since the article is pri- marily a narrative review, its main emphasis lies in presenting the current understanding of the ethnopharmacological and phyto- pharmacological significance of the Celtis genus. This focus is intended to facilitate future research and the acquisition of data for characterizing the genus and exploring its medicinal uses, with the potential to expedite the discovery of novel bioactive compounds.
Conclusion and futuristic prospects
Numerous findings show that plants of the Celtis genus have remarkable ethnopharmacological properties, thanks to their bio- logically active compounds. The three most investigated species, C. africana, C. australis, and C. tournefortii, have antibacterial, antioxidant, anticancer, and anti-inflammatory properties. Phytochemical studies revealed that the primary constituents occurring in this genus are amides, organic acids (phenolic acids, hydroxycinnamic acids, fatty acids, and aliphatic carboxylic acids), terpenoids (diterpenoids, triterpenoids, tocopherols, and carotenoids), flavonoids (flavanol, flavone, flavonol, and their glycosides), and esters (fatty acid esters).
Despite the important biological activities (antimicrobial, anticancer, and overall urease inhibition activities) of the genus Celtis, thanks to their potential new therapeutic molecules, this have not fully confirmed them because the studies were not fully established with scrutiny. Moreover, preliminary research has been limited to a few animal trials and is not widely accepted because it may behave differently in extensive studies.
To promote the therapeutic active compounds and as nutraceuticals of the Celtis genus, the research community may take some following steps.
- Because the activity of bioactive compounds in the Celtis genus is connected to their ethnopharmacological activity, retrace and organize traditional information about the Celtis species to understand how effective bioactive molecules may have been discovered.
- Further phytochemical analysis is needed to isolate compounds from the bio-active extracts and bioassay tests of the isolated compounds are also needed for the determination of the responsible phytochemicals. For example, the extracts C. australis and
C. africana showed some hope of having effective antimicrobial agents.
- Designing a new pathway to collect or synthesize target molecules noticed in Table 5 may lead to finding a novel medicinal molecule.
- In addition to the study of pharmacological activity, a pharmacokinetic study to evaluate the absorption, metabolism, distri- bution, and elimination of Celtis extract and its bioactive phytoconstituents is required.
- An accurate toxicology and dose-response graph are needed to indicate the therapeutic range which was missed in the maximum study.
- Clinical trials are required to evaluate the further biological consequences of these substances that have already been examined in vitro and in vivo.
- The bioactive agents’ safety and efficacy and the potential pathways of protection must also be evaluated before introducing
such molecules for further studies in human and animals.
Because of the up to date comprehensive information on the Celtis genus’ ethnopharmacological to potent bioactive molecules and phytochemistry, as well as the future prospects of the scope of Celtis genus research, this review article will be helpful to those who have an interest in the Celtis genus especially for its important ethnopharmacology and bioactive molecules.
Data availability statement
All the data involved in the review are explained in the manuscript.
CRediT authorship contribution statement
Md Abdus Samadd: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Vali- dation, Visualization, Writing – original draft. Md Jamal Hossain: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Miss Sharmin Zahan: Investigation, Methodology, Validation, Visualization, Writing – review & editing. Md Monirul Islam: Resources, Software, Validation, Visualization, Writing – review & editing. Mohammad A. Rashid: Investigation, Methodology,
Supervision, Validation, Visualization, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- R. Das, S. Mitra, A.M. Tareq, T.B. Emran, M.J. Hossain, A.M. Alqahtani, et al., Medicinal plants used against hepatic disorders in Bangladesh: a comprehensive review, J. Ethnopharmacol. 282 (2022) 114588.
- A.J. Chakraborty, T.M. Uddin, B.R.M. Zidan, S. Mitra, R. Das, F. Nainu, et al., Allium cepa: a Treasure of bioactive phytochemicals with prospective health benefits, Evid-Based Complement. Altern. Med. ECAM. 2022 (2022).
- S. Sarwar, M.J. Hossain, N.M. Irfan, T. Ahsan, M.S. Arefin, A. Rahman, et al., Renoprotection of selected antioxidant-rich foods (water Spinach and red Grape) and probiotics in gentamicin-induced nephrotoxicity and oxidative stress in rats, Life 12 (2022) 60.
- R. Das, M.S. Lami, A.J. Chakraborty, S. Mitra, T.E. Tallei, R. Idroes, et al., Ginkgo biloba: a Treasure of functional phytochemicals with multimedicinal applications, Evid. Based Complement Alternat. Med. 2022 (2022).
- S.A. Baba, M. Vahedi, I. Ahmad, B.S. Rajab, A.O. Babalghith, S. Irfan, et al., Crocus sativus L. tepal extract Induces apoptosis in human U87 glioblastoma cells, BioMed Res. Int. 2022 (2022).
- N. Anjum, M.J. Hossain, M.R. Haque, A. Chowdhury, M.A. Rashid, M.R. Kuddus, Phytochemical investigation of Schleichera oleosa (Lour.) Oken leaf, Bangladesh Pharm J. 24 (2021) 33–36.
- A.G. Atanasov, S.B. Zotchev, V.M. Dirsch, C.T. Supuran, Natural products in drug discovery: advances and opportunities, Nat. Rev. Drug Discov. 20 (2021) 200–216.
- M.F. Balandrin, A.D. Kinghorn, N.R. Farnsworth, Plant-Derived Natural Products in Drug Discovery and Development: an Overview, 1993.
- M. Riaz, N. Rasool, I.H. Bukhari, M. Shahid, M. Zubair, K. Rizwan, et al., In vitro antimicrobial, antioxidant, cytotoxicity and GC-MS analysis of
MazusGoodenifolius, Molecules 17 (2012) 14275–14278.
- I. Majeed, K. Rizwan, A. Ashar, T. Rasheed, R. Amarowicz, H. Kausar, et al., A comprehensive review of the ethnotraditional uses and biological and pharmacological potential of the genus mimosa, Int. J. Mol. Sci. 22 (2021) 7463.
- G. Velu, V. Palanichamy, A.P. Rajan, Phytochemical and pharmacological importance of plant secondary metabolites in modern medicine, Bioorg. Phase Nat. Food Overv. (2018) 135–156.
- N. Anjum, MdJ. Hossain, F. Aktar, M. Haque, M. Rashid, Kuddus MdR. Potential in vitro and in vivo bioactivities of schleichera oleosa (Lour.) Oken: a
traditionally important medicinal plant of Bangladesh, Res. J. Pharm. Technol. 15 (2022) 113–121, https://doi.org/10.52711/0974-360X.2022.00019.
- D.J. Newman, G.M. Cragg, Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019, J. Nat. Prod. 83 (2020) 770–803.
- S. Krief, C.M. Hladik, C. Haxaire, Ethnomedicinal and bioactive properties of plants ingested by wild chimpanzees in Uganda, J. Ethnopharmacol. 101 (2005)
1–15, https://doi.org/10.1016/j.jep.2005.03.024.
- R. Moffett, Sesotho Plant and Animal Names and Plants Used by the Basotho, UJ Press, 2010.
- S. Koduru, D.S. Grierson, A.J. Afolayan, Ethnobotanical information of medicinal plants used for treatment of cancer in the eastern Cape Province, South Africa, Curr. Sci. 92 (2007) 906–908.
- A.H. Scott, Zulu Medicinal Plants: an Inventory, University of Kwazulu Natal Press, 1996.
- B.-E. Van Wyk, B van Oudtshoorn, N. Gericke, Medicinal Plants of South Africa, Briza, 1997.
- R.D. Gaur, Flora of the District Garhwal, North West Himalaya, Transmedia, 1999.
- R.N. Chopra, S.L. Nayar, I.C. Chopra, Glossary of Indian medicinal plants (including the supplement), Council Sci. Ind Res New Delhi India (1986).
- J.A. Duke, E.S. Ayensu, Medicinal Plants of China, 1985.
- J.K. Maheshwari, B.S. Kalakoti, B. Lal, Ethnomedicine of Bhil Tribe of Jhabua district, m. P, Ancient Sci. Life 5 (1986) 255–261.
- A. Chevallier, The Encyclopedia of Medicinal Plants, 1996.
- C.R. Karnick, N.N. Pathak, Newer observations of folklore medicinal plants of Shiv-Khori forest area of the Western Himalayas, Naga 25 (1982) 159–162.
- N. Filali-Ansari, A. El Abbouyi, S. Khyari, Antioxidant Properties of Leaves and Seeds Hydromethanolic Extracts from Celtisaustralis, 2015, pp. 2834–2843.
- J. Lauriault, Identification Guide to the Trees of Canada, Fitzhenry & Whiteside, Markham, Ont, 1989.
- E.K. Nchabeleng, Determination of Biological Activity of Celtisafricana Extracts and its Endophytic Microflora and Mycoflora, University of, Johannesburg (South Africa), 2017.
- T.A. Mokoka, L.J. McGaw, J.N. Eloff, Antifungal efficacy of ten selected South African plant species against Cryptococcus neoformans, Pharm. Biol. 48 (2010) 397–404, https://doi.org/10.3109/13880200903150393.
- M.K. Laryea, L. Sheringham Borquaye, Antimalarial, antioxidant, and Toxicological evaluation of extracts of Celtisafricana, Grosseriavignei, Physalis micrantha, and Stachytarpheta angustifolia, Biochem Res Int 2021 (2021) e9971857, https://doi.org/10.1155/2021/9971857.
- S. Ahmad, R. Sharma, S. Mahajan, A. Gupta, Antibacterial activity of Celtisaustralis by in-vitro study, Int. J. Pharm. Pharmaceut. Sci. 4 (2012) 629–631.
- R. Badoni, D.K. Semwal, U. Rawat, Fatty acid composition and antimicrobial activity of Celtisaustralis L. fruits, J. Sci. Res. 2 (2010) 397–402.
- A. Ota, A.M. Viˇsnjevec, R. Vidrih, Zˇ. Prgomet, M. Neˇcemer, J. Hribar, et al., Nutritional, antioxidative, and antimicrobial analysis of the mediterranean
hackberry (Celtisaustralis L.), Food Sci. Nutr. 5 (2017) 160–170, https://doi.org/10.1002/fsn3.375.
- S. Keser, F. Keser, O. Kaygili, S. Tekin, I. Turkoglu, E. Demir, et al., Phytochemical compounds and biological activities of Celtistournefortii fruits, Anal Chem Lett 7 (2017) 344–355, https://doi.org/10.1080/22297928.2017.1329664.
- A. Baran, C. Keskin, Si Kandemir, Rapid biosynthesis of silver nanoparticles by Celtistournefortii LAM. leaf extract; investigation of antimicrobial and anticancer activities, Kahramanmaras¸ Sütçü I˙mam ÜniversitesiTarımVeDog˘aDerg 25 (2022) 72–84.
- C.L. Cantrell, N.H. Fischer, L. Urbatsch, M.S. McGuire, S.G. Franzblau, Antimycobacterial crude plant extracts from South, central, and North America,
Phytomedicine 5 (1998) 137–145.
- E.I. Rojas-Bedolla, J.L. Guti´errez-P´erez, M.I. Arenas-Lo´pez, M.M. Gonza´lez-Ch´avez, J.R. Zapata-Morales, C.L. Mendoza-Macías, et al., Chemical
characterization, pharmacological effects, and toxicity of an ethanol extract of CeltisPallidaTorr. (Cannabaceae) aerial parts, J. Ethnopharmacol. 219 (2018) 126–132, https://doi.org/10.1016/j.jep.2018.03.014.
- S. Perveen, A.M. Al-Taweel, G.A. Fawzy, A.M. El-Shafae, A. Khan, P. Proksch, Cytotoxic glucosphingolipid from Celtisafricana, Phcog. Mag. 11 (2015) S1–S5,
https://doi.org/10.4103/0973-1296.157662.
- R. Acquaviva, V. Sorrenti, R. Santangelo, V. Cardile, B. Tomasello, G. Malfa, et al., Effects of an extract of Celtisaetnensis (Tornab.) Strobl twigs on human colon cancer cell Cultures, Oncol. Rep. 36 (2016) 2298–2304.
- C.J.L. Murray, K.S. Ikuta, F. Sharara, L. Swetschinski, G.R. Aguilar, A. Gray, et al., Global Burden of bacterial antimicrobial resistance in 2019: a Systematic
analysis, Lancet 399 (2022) 629–655, https://doi.org/10.1016/S0140-6736(21)02724-0.
- N. Vaou, E. Stavropoulou, C. Voidarou, C. Tsigalou, E. Bezirtzoglou, Towards advances in medicinal plant antimicrobial activity: a review study on challenges and future perspectives, Microorganisms 9 (2021) 2041, https://doi.org/10.3390/microorganisms9102041.
- A.H. Cheung Lam, N. Sandoval, R. Wadhwa, J. Gilkes, T.Q. Do, W. Ernst, et al., Assessment of free fatty acids and cholesteryl esters delivered in liposomes as novel class of antibiotic, BMC Res. Notes 9 (2016) 337, https://doi.org/10.1186/s13104-016-2138-8.
- M.K. Yadav, S.-W. Chae, G.J. Im, J.-W. Chung, J.-J. Song, Eugenol: a phyto-compound effective against methicillin-resistant and methicillin-sensitive
Staphylococcus aureus clinical strain biofilms, PLoS One 10 (2015) e0119564, https://doi.org/10.1371/journal.pone.0119564.
- K. Benamar, S.I. Koraichi, K. Fikri-Benbrahim, Ethnobotany, phytochemistry and pharmacological activities of Celtisaustralis: a review, J. HerbmedPharmacol. 12 (2023).
- F.T. Bonner, The Woody Plant Seed Manual, Forest Service, 2008.
- A. Sattarian, Contribution to the Biosystematics of Celtis L. (Celtidaceae) with Special Emphasis on the African Species, 2006.
- G. Kozlowski, Genetic Diversity and Conservation of Woody Species, 2021.
- W.H. Duncan, M.B. Duncan, Trees of the Southeastern United States, University of Georgia Press, 2000.
- ITIS – Report: Celtis. [cited 7 November 2022]. Available: https://www.itis.gov/servlet/SingleRpt/SingleRpt?search_topic=TSN&search_value=19039#null.
- L. Poorter, Biodiversity of West African Forests: an Ecological Atlas of Woody Plant Species, CABI, 2004.
- M.A. Hyde, B.T. Wursten, P. Ballings, C.M. Palgrave, Flora of Zimbabwe: Species Information, Cardiospermumhalicacabum, 2011.
- E. Schmidt, M. Lotter, W. McCleland, Trees and Shrubs of Mpumalanga and Kruger National Park, Jacana Media, 2002.
- T. El-Alfy, H. El-Gohary, N. Sokkar, Tawab S. Abdel, D. Al-Mahdy, Botanical and genetic characteristics of Celtisaustralis L. and Celtisoccidentalis L. grown in Egypt, Bull. Fac. Pharm. Cairo Univ. 49 (2011) 37–57, https://doi.org/10.1016/j.bfopcu.2011.07.007.
- C.M. Herrera, Vertebrate-dispersed plants of the Iberian Peninsula: a study of fruit characteristics, Ecol. Monogr. 57 (1987) 305–331, https://doi.org/10.2307/
- D. Magni, G. Caudullo, Celtisaustralis in Europe: Distribution, Habitat, Usage and Threats, 2016.
- Celtisjessoensis – Trees and Shrubs Online. [cited 7 September 2023]. Available: https://www.treesandshrubsonline.org/articles/celtis/celtis-jessoensis/.
- A.E. Radford, H.E. Ahles, C.R. Bell, Manual of the Vascular Flora of the Carolinas, Univ of North Carolina Press, 2010.
- H.A. Stephens, Woody plants of the North central Plains, Woody Plants North Cent Plains (1973) [cited 7 Sep 2023]. Available: https://www.cabdirect.org/ cabdirect/abstract/19750627207.
- R.E. Woodson, R.W. Schery, Flora of Panama, Ann. Mo. Bot. Gard. 67 (1980) ii–xxxiii, https://doi.org/10.2307/2398968.
- L. Shultz, Magnoliophyta: Magnoliidae and Hamamelidae, Flora N Am. North Mex. 3 (1997).
- E. Soepadmo, Ulmaceae, Flora Malesiana – Ser. 1 Spermatophyta 8 (1974) 31–76.
- Z. Wu, P.H. Raven, D. Hong, Flora of China, in: Ulmaceae through Basellaceae, vol. 5, Science Press, 2003.
- W. ZhengYi, P.H. Raven, H. DeYuan, Flora of China. Volume 5: Ulmaceae through Basellaceae, Flora China 5 UlmaceaeBasellaceae (2003).
- J. Zielin´ski, A. Petrova, R. Natcheva, New Species for the Bulgarian Flora, 2012.
- R.M. Polhill, Ulmaceae. Crown Agents for Oversea Governments and Administrations, 1966.
- Celtisadolfi-fridericii. [cited 15 September 2023]. Available: https://prota.prota4u.org/protav8.asp?h M4&t Celtis,adolfi-fridericii&p Celtis adolfi- fridericii#Synonyms.
- G. Dali, A. Diame, To, Ethnobotany and Ecological Studies of Plants Used for Reproductive Health: A Case Study at Bia Biosphere Reserve in the Western Region of Ghana, University of Cape Coast Ghana Press, 2010.
- A. Dyer, The Flowering Plants of Africa, No121, 1955.
- H.G. Kim, S. Choi, J. Lee, Y.H. Hong, D. Jeong, K. Yoon, et al., Src is a prime target inhibited by Celtischoseniana methanol extract in its anti-inflammatory action, Evid. Based Complement Alternat. Med. 2018 (2018) e3909038, https://doi.org/10.1155/2018/3909038.
- J.C.T. Uphof, Dictionary of Economic Plants, J. Cramer, Weinheim, 1959.
- B.S. Adhikari, M.M. Babu, P.L. Saklani, G.S. Rawat, Medicinal plants diversity and their conservation status in Wildlife Institute of India (WII) Campus, Dehradun. EthnobotLeafl. 2010 (2010) 6.
- M. Ajaib, Z. Khan, Ethnobotanical studies of useful trees of district Kotli, Azad Jammu and Kashmir, Biologia (Bratisl). 60 (2014) 63–71.
- A.P. Singh, An appraisal of the concepts of health and disease in the Folk cultures of Uttarakhand Himalaya, Coll. Antropol. 6 (2) (1982 Jan 1) 229–231.
- Hs-Ha-G Mujtaba, S.-A. Majid, Ethnomedicinal potential of the tree species of district Abbottabad-Pakistan, Proc. Abstr. (2013) 94.
- V.E.G. Rodrigues, D.A. de Carvalho, Semidecidual Na Regiao Do Alto Rio Grande Minas Gerais, vol. 14, 2008.
- W.B. Mors, C.T. Rizzini, N.A. Pereira, Medicinal Plants of Brazil, Reference Publications, Inc, 2000.
- P. Hanelt, Mansfeld’s world database of agricultural and horticultural crops, Mansfelds World Database Agric. Hortic Crops (2017).
- V. Tene, O. Malago´n, P.V. Finzi, G. Vidari, C. Armijos, T. Zaragoza, An ethnobotanical survey of medicinal plants used in Loja and Zamora-Chinchipe, Ecuador, J. Ethnopharmacol. 111 (2007) 63–81, https://doi.org/10.1016/j.jep.2006.10.032.
- E. Hernandez-Galicia, A. Aguilar-Contreras, L. Aguilar-Santamaría, R. Roman-Ramos, A. Chavez-Miranda, L. Garcia-Vega, et al., Studies on Hypoglycemic activity of Mexican medicinal plants, Proc. West. Pharmacol. Soc. 45 (2002) 118–124.
- CSP da Silva, C.E.B. Proença, UsoE Disponibilidade De RecursosMedicinais No Município De Ouro Verde de Goia´s, GO, Brasil, Acta Bot. Bras. 22 (2008)
481–492, https://doi.org/10.1590/S0102-33062008000200016.
- N.Z. Gonçalves, R.S. Lino Júnior, C.R. Rodrigues, A.R. Rodrigues, L.C. Cunha, Acute oral toxicity of Celtisiguanaea (Jacq.) Sargent leaf extract (Ulmaceae) in
rats and mice, Rev. Bras. Plantas Med. 17 (2015) 1118–1124, https://doi.org/10.1590/1983-084X/14_128.
- D.E. Moerman, Native American Ethnobotany, Timber Press, Portland OR, 1998.
- U. Quattrocchi, CRC World Dictionary of Medicinal and Poisonous Plants: Common Names, Scientific Names, Eponyms, Synonyms, and Etymology (5 Volume Set), CRC Press, 2012.
- C.W. Choi, S.B. Song, J.S. Oh, Y.H. Kim, Antiproliferation effects of selected Tanzania plants, Afr. J. Tradit., Complementary Altern. Med. 12 (2015) 96–102.
- International Collation of Traditional and Folk Medicine, International collation of traditional and folk medicine, World Sci. (1997) 1–211, https://doi.org/ 10.1142/9789812819482_0001.
- T. Nole, T.D.W. Lionel, T.F.S. Cedrix, A.A. Gabriel, Ethnomedical and ethnopharmacological study of plants used for potential treatments of diabetes and arterial hypertension by indigenous people in three phytogeographic regions of Cameroon, Diabetes Case Rep. 1 (2016) 1–9.
- O.P. Tjeck, A. Souza, P. Mickala, A.N. Lepengue, B. M’Batchi, Bio-efficacy of medicinal plants used for the management of diabetes mellitus in Gabon: an ethnopharmacological approach, J. Intercult. Ethnopharmacol. 6 (2017) 206–217, https://doi.org/10.5455/jice.20170414055506.
- V.P. Kamboj, B.S. Setty, N.M. Khanna, Semen ‘Coagulation’ — a potential approach to contraception, Contraception 15 (1977) 601–610, https://doi.org/
- N.P. Manandhar, Plants and people of Nepal, Plants People Nepal (2002).
- J. Buragohain, Folk medicinal plants used in gynecological disorders in Tinsukia district, Assam, India, Fitoterapia 79 (2008) 388–392.
- E.O. Okpala, P.A. Onocha, M.S. Ali, S.Z.- Ur-Rehmen, M. Lateef, Zenkeramide: a new iso-benzofuranone propanamide and urease inhibitory constituents of
Celtiszenkeri EnglStem bark (Ulmaceae), Nat. Prod. Res. 37 (2021) 93–98, https://doi.org/10.1080/14786419.2021.1954643.
- R.W.J. Keay, Trees of Nigeria, Clarendon Press, 1989.
- O.A. Ugbogu, T.O. Oyebola, Checklist of medicinal plants in Omo forest Reserve in the Rain forest Zone, J for Res Manag 10 (2013) 107–119.
- J.K. Maheshwari, Taxonomic studies on Indian Guttiferae II. The genus Mesua Linn, Nelumbo 5 (1963) 335–343.
- A.J. Seukep, J.A.K. Noumedem, D.E. Djeussi, V. Kuete, 9 – Genotoxicity and teratogenicity of african medicinal plants, in: V. Kuete (Ed.), Toxicological Survey of African Medicinal Plants, Elsevier, 2014, pp. 235–275, https://doi.org/10.1016/B978-0-12-800018-2.00009-1.
- * M. Saxena, J. Saxena, R. Nema, D. Singh, A. Gupta, Phytochemistry of medicinal plants, J. Pharmacogn. Phytochem. 1 (2013) 168–182.
- S. Fedha, S. Omondi, Phytochemical analysis of some selected plants and families in the University botanic Garden of Maseno, Kenya, IOSR J. Pharm. Biol. Sci.
12 (2017) 31–38, https://doi.org/10.9790/3008-1204023138.
Rendle, Chem Data Collect 28 (2020) 100483.
- Q. Wei, W. Guo, Chemical components of volatile Oil from leaves and stems of Celtissinensis pers, J. Essent Oil Bear Plants 23 (2020) 772–778, https://doi.org/ 10.1080/0972060X.2020.1794984.
- E.O. Okpala, P.A. Onocha, M.S. Ali, Antioxidant activity of phytol dominated stem bark and leaf essential oils of Celtiszenkeri Engl, Trends Phytochem. Res. 6 (2022) 137–144, https://doi.org/10.30495/tpr.2022.1952985.1246.
- A.G. Ayanlowo, Z. Gara´di, I. Boldizs´ar, A. Darcsi, A.N. Nedves, B. Varjas, et al., UHPLC-DPPH method reveals antioxidant tyramine and octopamine derivatives
in Celtisoccidentalis, J. Pharm. Biomed. Anal. 191 (2020) 113612.
- D.K. Kim, J.P. Lim, J.W. Kim, H.W. Park, J.S. Eun, Antitumor and antiinflammatory constituents from Celtissinensis, Arch Pharm. Res. (Seoul) 28 (2005) 39–43, https://doi.org/10.1007/BF02975133.
- A.M. Al-Taweel, S. Perveen, A.M. El-Shafae, G.A. Fawzy, A. Malik, N. Afza, et al., Bioactive phenolic amides from Celtisafricana, Molecules 17 (2012) 2675–2682.
- K.J. Jumeta, D.U. KenouKagho, J.E. Terence Ateba, Y.S. FongangFotsing, J.J. KezetasBankeu, N. Sewald, et al., A new cerebroside and bioactive compounds from Celtisadolphi-friderici Engl. (Cannabaceae), Biochem. Systemat. Ecol. 94 (2021) 104201, https://doi.org/10.1016/j.bse.2020.104201.
- D.K. Kim, K. Lee, Inhibitory effect of Trans-N-p-coumaroyl tryamine from the twigs of Celtischinensis on the acetylcholinesterase, Arch Pharm. Res. (Seoul) 26
(2003) 735–738, https://doi.org/10.1007/BF02976684.
- Sj Abdulwahid-Kurdi, Detect polyphenol and fatty acid content of two wild plants collected in Mazne Sub-district, Kurdistan region of Iraq, Curr. Res. Nutr. Food Sci. 11 (2023).
- M.D. Ayoola, A.S. Odediran, S.O. Famuyiwa, M. Oluwagbemi, L.I. Afolabi, F.A. Oladoja, et al., Evaluation of the Antihyperglycaemic Activities, Safety and Phytochemical Profile of Celtiszenkeri Engl, 2023.
- B. Zanchet, D. Miorando, D.B. Gomes, G. Locateli, C.A.D. Vecchia, P.Z. Serpa, et al., In vitro antiproliferative potential of Celtisiguanaea against ovarian (OVCAR-3) and colon (HT-29) tumor cell, Eur. J. Med. Plants (2019) 1–9, https://doi.org/10.9734/ejmp/2019/v30i330177.
- M. da S. Vargas, Investigation of the chemical composition and antioxidant capacity of extracts from the leaves of Celtisehrenbergiana [cited 7 Sep 2023]. Available: https://repositorio.unipampa.edu.br/jspui/handle/riu/5521, 2020.
- F. Safari, H. Hassanpour, A. Alijanpour, Evaluation of hackberry (CeltisaustralisL.) fruits as sources of bioactive compounds, Sci. Rep. 13 (2023) 12233, https:// doi.org/10.1038/s41598-023-39421-x.
- V. Sommavilla, D. Haidacher-Gasser, M. Sgarbossa, C. Zidorn, Seasonal variation in phenolics in leaves of Celtisaustralis (Cannabaceae), Biochem. Systemat.
Ecol. 41 (2012) 110–114, https://doi.org/10.1016/j.bse.2011.12.028.
- FroederAlf. EstudoFitoquímico E De Toxicidade (Aguda E Subaguda), De Celtisiguanaea (Jacq.) Sarg. EmRatosWistar, 2015. Available: https://www. semanticscholar.org/paper/ESTUDO-FITOQU%C3%8DMICO-E-DE-TOXICIDADE-(AGUDA-E-DE-em-Froeder/cf3aed757ccc8153d8f839006baad899d3c07e22.
- I. Yıldırım, Y. Ug˘ur, T. Kutlu, Investigation of antioxidant activity and phytochemical compositions of Celtistournefortii, Free RadicAntioxid 7 (2017) 160–165,
https://doi.org/10.5530/fra.2017.2.24.
- I.H. Gecibesler, Antioxidant activity and phenolic Profile of Turkish Celtistournefortii, Chem. Nat. Compd. 55 (2019) 738–742.
- B.Y. Hwang, H.-B. Chai, L.B.S. Kardono, S. Riswan, N.R. Farnsworth, G.A. Cordell, et al., Cytotoxic triterpenes from the twigs of Celtisphilippinensis, Phytochemistry 62 (2003) 197–201, https://doi.org/10.1016/S0031-9422(02)00520-4.
- P. Seephonkai, R. Ishikawa, M.A. Arai, T. Kowithayakorn, T. Koyano, M. Ishibashi, New flavanol dimers from the bark of Celtistetrandra and their TRAIL resistance-overcoming activity, Nat. Prod. Commun. 13 (2018) 1934578X1801300412, https://doi.org/10.1177/1934578X1801300412.
- H.M. Cuchillo, D.C. Puga, N. Wrage-Mo¨nning, M.J.G. Espinosa, B.S. Montan˜o, A. Navarro-Ocan˜a, et al., Chemical composition, antioxidant activity and bioactive compounds of vegetation species ingested by goats on semiarid rangelands, J. Anim. Feed Sci. 22 (2013) 106–115.
- E. Taxonomic Ahmed, Phytochemical and Biological Screening of Some Selected Medicinal Plants of Lesser Himalaya Pakistan, PMAS-Arid Agriculture University, Rawalpindi, 2018. PhD Thesis.
- R. Badoni, D.K. Semwal, U. Rawat, G.J.P. Singh, Celtisanin, a novel sulphonated phenolic from Celtisaustralis L. Fruits, Nat. Prod. Res. 24 (2010) 1282–1286.
- T. Perovi´c, J. Petrovi´c, U. Gaˇsi´c, M. Kosti´c, A. C´iri´c, Natural extracts against agricultural pathogens: a case study of Celtisaustralis L, Food Sci. Nutr. 11 (2023)
3358–3364, https://doi.org/10.1002/fsn3.3325.
- B. Zanchet, D.B. Gomes, V.S. Corralo, K.A.P. Diel, Faust C. Scho¨nellAP, et al., Effects of hydroalcoholic extract of Celtisiguanaea on markers of cardiovascular
diseases and glucose metabolism in cholesterol-fed rats, Rev. Bras. Farmacogn 28 (2018) 80–91, https://doi.org/10.1016/j.bjp.2017.12.001.
- T.S. El-Alfy, H.M.A. El-Gohary, N.M. Sokkar, M. Hosny, D.A. Al-Mahdy, A new flavonoid C-glycoside from Celtisaustralis L. and Celtisoccidentalis L. leaves and
potential antioxidant and cytotoxic activities, Sci. Pharm. 79 (2011) 963–975, https://doi.org/10.3797/scipharm.1108-19.
- S. Perveen, A.M. El-Shafae, A. Al-Taweel, G.A. Fawzy, A. Malik, N. Afza, et al., Antioxidant and urease inhibitory C-glycosylflavonoids from Celtisafricana,
J. Asian Nat. Prod. Res. 13 (2011) 799–804, https://doi.org/10.1080/10286020.2011.593171.
- Y. Zhang, Z. Qi, W. Wang, L. Wang, F. Cao, L. Zhao, et al., Isovitexin inhibits Ginkgolic acids-induced inflammation through downregulating SHP2 activation, Front. Pharmacol. 12 (2021).
- A. Baran, C. Keskin, Determination of constituents of extract of Celtistournefortii Lam. By LC-MS/MS, investigation of enzyme inhibition, antimicrobial and anticancer effects, Int J Pure Appl Sci. 9 (2023) 56–65, https://doi.org/10.29132/ijpas.1168200.
- M.A.K. Lodhi, E.L. Rice, Allelopathic effects of Celtis laevigata, Bull. Torrey Bot. Club 98 (1971) 83–89, https://doi.org/10.2307/2483771.
- N. Filali-Ansari, A. El Abbouyi, A. Kijjoa, S. El Maliki, S. Khyari, Antioxidant and Antimicrobial Activities of Chemical Constituents from Celtisaustralis, vol. 8, 2016, pp. 338–347.
- R. Badoni, D.K. Semwal, P.P. Badoni, S.K. Kothiyal, U. Rawat, A novel bacteriohopanoid from CeltisaustralisL. Bark, Chin. Chem. Lett. 22 (2011) 81–84.
- R.R. Trevisan, C.P. Lima, C.M.S. Miyazaki, F.A. Pesci, C.B. Silva, B.C.K. Hirota, et al., Evaluation of the phytotoxic activity focused on the Allelopathic effect of
the extract from the bark ofCeltisiguanaea (Jacq.) Sargent Ulmaceae and purification of two terpenes, Rev. Bras. Plantas Med. 14 (2012) 494–499, https://doi. org/10.1590/S1516-05722012000300011.
- D. Egamberdieva, N. Mamedov, E. Ovidi, A. Tiezzi, L. Craker, Phytochemical and pharmacological properties of medicinal plants from Uzbekistan: a review, J Med Act Plants 5 (2017) 59–75.
- A.L. Tariq, A.L. Reyaz, Significances and importance of phytochemical present in Terminalia chebula, Int. J. Drug Dev. Res. 5 (2013) 256–262.
- A. Wadood, Phytochemical analysis of medicinal plants occurring in Local area of Mardan, Biochem. Anal. Biochem. (2013) 2, https://doi.org/10.4172/2161- 1009.1000144.
- M.B. Sporn, A.B. Roberts, D.S. Goodman, The Retinoids: Biology, Chemistry, and Medicine, Raven Press, 1994.
- E.O. Okpala, P.A. Onocha, M.S. Ali, S.Z.- Ur-Rehmen, M. Lateef, Zenkeramide: a new iso-BenzofuranonePropanamide and urease inhibitory constituents of
Celtiszenkeri EnglStem bark (Ulmaceae), Nat. Prod. Res. 37 (2021) 93–98, https://doi.org/10.1080/14786419.2021.1954643.
- S. Thomas, Hypoglycemic and Hypolipidemic effect of Celtisphilippensis Blanco on albino Wistar rats, NVEO-Nat. Volatiles Essent Oils J NVEO (2022) 1294–1300.
- A. Khan, A.M. Al-Taweel, S. Perveen, G.A. Fawzy, A.H. Gilani, Studies on prokinetic, laxative, antidiarrheal and gut modulatory activities of the aqueous- methanol extract of Celtisafricana and underlying mechanisms, Int. J. Pharmacol. 8 (8) (2012) 701–707, https://doi.org/10.3923/ijp.2012.701.707.
- R. B Semwal, D. K Semwal, Analgesic and anti-inflammatory activities of extracts and fatty acids from Celtisaustralis L, Nat. Prod. J. 2 (2012) 323–327.
- N. Filali-Ansari, A. El Abbouyi, S. Khyari, R. Eddoha, Antibacterial and antifungal activities of seeds and leaves extracts from Celtisaustralis, J. Chem. Biol. Phys. Sci. 5 (2015) 1401–1407.
- F.B. de Sousa, J.L.R. Martins, I.F. Florentino, R.O. do Couto, M.V.M. Nascimento, P.M. Galdino, et al., Preliminary studies of gastroprotective effect ofCeltisiguanaea (Jacq.) Sargent leaves (Ulmaceae), Nat. Prod. Res. 27 (2013) 1102–1107, https://doi.org/10.1080/14786419.2012.698407.
- J.L.R. Martins, F.B. Sousa, J.O. Fajemiroye, P.C. Ghedini, P.M. Ferreira, E.A. Costa, Anti-ulcerogenic and antisecretory effects of Celtisiguanaea (Jacq.) Sargent hexane leaf extract, Rev. Bras. Plantas Med. 16 (2014) 250–255.
- A.E. Abbouyi, S. Echarrafi, N. Filali-Ansari, S.E. Khyari, Wound healing potential of ethyl acetate of seeds extract from Celtisaustralis, Int. J. Pharmaceut. Chem. Biol. Sci. 5 (2015).
- M.A. Temiz, A. Temur, Y. Akgeyik, A. Uyar, Protective Effect of Celtistournefortii against Copper-Induced Toxicity in Rat Liver, 2021, https://doi.org/10.2754/ avb202190010091.
- M. Temiz, A. Temur, M. Sürücü, T. Yaman, Antioxidant and Hepatoprotective Effect of Oriental Hackberry (Celtistournefortii L.) against CCL4 Injury in Rat Liver, 2019.
- A.Z.M. Salem, A.E. Kholif, M.M.Y. Elghandour, S.R. Hernandez, I.A. Domínguez-Vara, M. Mellado, Effect of increasing levels of seven tree species extracts added to a high concentrate diet on in-vitro Rumen gas output, Anim. Sci. J. 85 (2014) 853–860, https://doi.org/10.1111/asj.12218.
- T. Thompson, The staggering death toll of drug-resistant bacteria, Nature (2022), https://doi.org/10.1038/d41586-022-00228-x. (Accessed 9 September 2023).
- G. Casillas-Vargas, C. Ocasio-Malav´e, S. Medina, C. Morales-Guzm´an, R.G. Del Valle, N.M. Carballeira, et al., Antibacterial fatty acids: an update of possible
- E.P. Ivanova, S.H. Nguyen, Y. Guo, V.A. Baulin, H.K. Webb, V.K. Truong, et al., Bactericidal activity of self-assembled palmitic and stearic fatty acid crystals on highly ordered Pyrolytic graphite, Acta Biomater. 59 (2017) 148–157, https://doi.org/10.1016/j.actbio.2017.07.004.
- J.B. Parsons, J. Yao, M.W. Frank, P. Jackson, C.O. Rock, Membrane disruption by antimicrobial fatty acids releases low-molecular-weight proteins from
Staphylococcus aureus, J. Bacteriol. 194 (2012) 5294–5304, https://doi.org/10.1128/jb.00743-12.
- J.G. Kenny, D. Ward, E. Josefsson, I.-M. Jonsson, J. Hinds, H.H. Rees, et al., The Staphylococcus aureus response to unsaturated long chain free fatty acids: survival mechanisms and virulence implications, PLoS One 4 (2009) e4344, https://doi.org/10.1371/journal.pone.0004344.
- C.J. Zheng, J.-S. Yoo, T.-G. Lee, H.-Y. Cho, Y.-H. Kim, W.-G. Kim, Fatty acid synthesis is a target for antibacterial activity of unsaturated fatty acids, FEBS Lett. 579 (2005) 5157–5162, https://doi.org/10.1016/j.febslet.2005.08.028.
- S.W. Jung, S. Thamphiwatana, L. Zhang, M. Obonyo, Mechanism of antibacterial activity of liposomal linolenic acid against Helicobacter pylori, PLoS One 10 (2015) e0116519, https://doi.org/10.1371/journal.pone.0116519.
- S. Abbaszadeh, A. Sharifzadeh, H. Shokri, A.R. Khosravi, A. Abbaszadeh, Antifungal efficacy of thymol, carvacrol, eugenol and menthol as alternative agents to
control the growth of food-relevant Fungi, J MycolMedicale 24 (2014) e51–e56.
pathogen Helicobacter pylori, Ann. Clin. Microbiol. Antimicrob. 4 (2005) 1–7.
- K.-W. Jeong, J.-Y. Lee, D.-I. Kang, J.-U. Lee, S.Y. Shin, Y. Kim, Screening of flavonoids as candidate antibiotics against Enterococcus faecalis, J. Nat. Prod. 72 (2009) 719–724, https://doi.org/10.1021/np800698d.
- A.R. Brown, K.A. Ettefagh, D. Todd, P.S. Cole, J.M. Egan, D.H. Foil, et al., A mass spectrometry-based assay for improved quantitative measurements of efflux pump inhibition, PLoS One 10 (2015) e0124814.
- A. Klanˇcnik, M. Sˇiki´cPogaˇcar, K. Troˇst, M. TuˇsekZˇnidariˇc, B. MozetiˇcVodopivec, S. SmoleMoˇzina, Anti-Campylobacter activity of Resveratrol and an extract
from waste pinot noir grape skins and seeds, and resistance of Camp. Jejuni Planktonic and biofilm cells, mediated via the Cmeabc efflux pump, J. Appl. Microbiol. 122 (2017) 65–77, https://doi.org/10.1111/jam.13315.
- D. Lechner, S. Gibbons, F. Bucar, Plant phenolic compounds as Ethidium Bromide efflux inhibitors in Mycobacterium smegmatis, J. Antimicrob. Chemother. 62 (2008) 345–348, https://doi.org/10.1093/jac/dkn178.
- C. Morel, F.R. Stermitz, G. Tegos, K. Lewis, Isoflavones as potentiators of antibacterial activity, J. Agric. Food Chem. 51 (2003) 5677–5679, https://doi.org/
- H.K. Randhawa, K.K. Hundal, P.N. Ahirrao, S.M. Jachak, H.S. Nandanwar, Efflux pump inhibitory activity of flavonoids isolated from Alpiniacalcarata against methicillin-resistant Staphylococcus aureus, Biologia (Bratisl). 71 (2016) 484–493, https://doi.org/10.1515/biolog-2016-0073.
- D. Wu, Y. Kong, C. Han, J. Chen, L. Hu, H. Jiang, et al., d-Alanine:d-alanine ligase as a new target for the flavonoids quercetin and apigenin, Int. J. Antimicrob.
Agents 32 (2008) 421–426, https://doi.org/10.1016/j.ijantimicag.2008.06.010.
- S.S. Kang, J.-G. Kim, T.-H. Lee, K.-B. Oh, Flavonols inhibit Sortases and Sortase-mediated Staphylococcus aureus clumping to Fibrinogen, Biol. Pharm. Bull. 29 (2006) 1751–1755, https://doi.org/10.1248/bpb.29.1751.
- R. Paduch, M. Kandefer-Szerszen´, M. Trytek, J. Fiedurek, Terpenes: substances useful in human healthcare, Arch. Immunol. Ther. Exp. 55 (2007) 315–327,
https://doi.org/10.1007/s00005-007-0039-1.
strains, Ann. Clin. Microbiol. Antimicrob. 10 (2011) 1–6.
- M. Broniatowski, P. Mastalerz, M. Flasin´ski, Studies of the Interactions of Ursane-type bioactive terpenes with the model of Escherichia coli inner membrane—Langmuir monolayer approach, BiochimBiophys. Acta BBA-Biomembr. 1848 (2015) 469–476.
- A. Chanvitan, S. Ubolcholket, V. Chongsuvivatwong, A. Geater, Risk factors for Squamous cell carcinoma in southern Thailand, Esophageal. Canver Stud. South
- Y.E.M. Dommels, M.M.G. Haring, N.G.M. Keestra, G.M. Alink, P.J. van Bladeren, B. van Ommen, The role of cyclooxygenase in N-6 and N-3 Polyunsaturated
fatty acid mediated effects on cell proliferation, PGE 2 synthesis and cytotoxicity in human Colorectal carcinoma cell lines, Carcinogenesis 24 (2003) 385–392, https://doi.org/10.1093/carcin/24.3.385.
- D.F. Horrobin, V.A. Ziboh, The importance of Linoleic acid metabolites in cancer metastasis and in the synthesis and actions of 13-HODE, Adv. Exp. Med. Biol. 433 (1997) 291–294, https://doi.org/10.1007/978-1-4899-1810-9_61.
- H. Harada, U. Yamashita, H. Kurihara, E. Fukushi, J. Kawabata, Y. Kamei, Antitumor activity of palmitic acid found as A selective cytotoxic substance in A
marine red Alga, Anticancer Res. 22 (2002) 2587–2590.
- M. Jo´´zwiak, A. Filipowska, F. Fiorino, M. Struga, Anticancer activities of fatty acids and their heterocyclic derivatives, Eur. J. Pharmacol. 871 (2020) 172937, https://doi.org/10.1016/j.ejphar.2020.172937.
- H.E. Khalil, H.-I.M. Ibrahim, E.A. Ahmed, P.M. Emeka, I.A. Alhaider, Orientin, a bio-flavonoid from Trigonellahamosa L., regulates COX-2/PGE-2 in A549 cell lines via miR-26b and miR-146a, Pharmaceuticals 15 (2022) 154, https://doi.org/10.3390/ph15020154.
- C.V. Rao, H.L. Newmark, B.S. Reddy, Chemopreventive effect of squalene on colon cancer, Carcinogenesis 19 (1998) 287–290.
- K.K. Auyeung, Q.-B. Han, J.K. Ko, Astragalus membranaceus: a review of its protection against inflammation and gastrointestinal cancers, Am. J. Chin. Med. 44
- S.Y. Park, R. Seetharaman, M.J. Ko, D.Y. Kim, T.H. Kim, M.K. Yoon, et al., Ethyl linoleate from garlic attenuates lipopolysaccharide-induced pro-inflammatory
cytokine production by inducing heme oxygenase-1 in RAW264.7 cells, Int. Immunopharm. 19 (2014) 253–261, https://doi.org/10.1016/j. intimp.2014.01.017.
- S.M. Imtiaz, A. Aleem, F. Saqib, A.N. Ormenisan, A. Elena Neculau, C.V. Anastasiu, The potential involvement of an ATP-dependent potassium channel- opening mechanism in the smooth muscle relaxant properties of Tamarixdioica Roxb, Biomolecules 9 (2019) 722, https://doi.org/10.3390/biom9110722.
- T. Khan, S. Ali, R. Qayyum, I. Hussain, F. Wahid, A.J. Shah, Intestinal and vascular smooth muscle relaxant effect of viscum album explains its medicinal use in hyperactive gut disorders and hypertension, BMC Compl. Alternative Med. 16 (2016) 251, https://doi.org/10.1186/s12906-016-1229-3.
- H.B. Sahoo, R. Sagar, A. Kumar, A. Bhaiji, S.K. Bhattamishra, Antidiarrhoeal investigation of Apiumleptophyllum (pers.) by modulation of Na+K+ATPase, nitrous
oxide and intestinal transit in rats, Biomed. J. 39 (2016) 376–381, https://doi.org/10.1016/j.bj.2016.11.003.
- M.B. Colovic, D.Z. Krstic, T.D. Lazarevic-Pasti, A.M. Bondzic, V.M. Vasic, Acetylcholinesterase inhibitors: pharmacology and toxicology, Curr. Neuropharmacol. 11 (2013) 315–335.
- H.L. Mobley, R.P. Hausinger, Microbial ureases: significance, regulation, and molecular characterization, Microbiol. Rev. 53 (1989) 85–108, https://doi.org/
- H.L. Mobley, M.D. Island, R.P. Hausinger, Molecular biology of microbial ureases, Microbiol. Rev. 59 (1995) 451–480, https://doi.org/10.1128/mr.59.3.451- 480.1995.
- A.Y. Lee, M.H. Lee, S. Lee, E.J. Cho, Neuroprotective effect of alpha-linolenic acid against Aβ-mediated inflammatory responses in C6 Glial cell, J. Agric. Food Chem. 66 (2018) 4853–4861, https://doi.org/10.1021/acs.jafc.8b00836.
- J.M. Lou-Bonafonte, R. Martínez-Beamonte, T. Sanclemente, J.C. Surra, L.V. Herrera-Marcos, J. Sanchez-Marco, et al., Current insights into the biological action of squalene, Mol. Nutr. Food Res. 62 (2018) 1800136, https://doi.org/10.1002/mnfr.201800136.
- S. Pengnet, S. Prommaouan, P. Sumarithum, W. Malakul, Naringin reverses high-cholesterol diet-induced Vascular dysfunction and oxidative stress in rats via regulating LOX-1 and NADPH oxidase subunit expression, BioMed Res. Int. 2019 (2019) e3708497, https://doi.org/10.1155/2019/3708497.
- W.-Y. Wu, Y.-K. Cui, Y.-X. Hong, Y.-D. Li, Y. Wu, G. Li, et al., Doxorubicin cardiomyopathy is Ameliorated by Acacetin via Sirt1-mediated activation of AMPK/ Nrf2 signal molecules, J. Cell Mol. Med. 24 (2020) 12141–12153, https://doi.org/10.1111/jcmm.15859.
- X. Zhang, P. Zhu, X. Zhang, Y. Ma, W. Li, J.-M. Chen, et al., Natural antioxidant-Isoliquiritigenin Ameliorates contractile dysfunction of Hypoxic Cardiomyocytes via AMPK signaling pathway, Mediat. Inflamm. 2013 (2013) e390890, https://doi.org/10.1155/2013/390890.
- J. Grassmann, Terpenoids as plant antioxidants, Vitam. Horm. 72 (2005) 505–535, https://doi.org/10.1016/S0083-6729(05)72015-X.
- A.A. Adedapo, F.O. Jimoh, A.J. Afolayan, P.J. Masika, Antioxidant properties of the methanol extracts of the leaves and stems of Celtisafricana, Record Nat. Prod. 3 (2009).
- M. Shokrzadeh, H bakhshi Jouybari, M. Hosseinpour, A. Ziar, E. Habibi, Antioxidant and protective effect of hydroalcoholic extract of Celtisaustralis L. On CCl4 induced hepatotoxicity, Pharm. Biomed. Res.. (2019), https://doi.org/10.18502/pbr.v4i3.541. (Accessed 7 September 2023).
- R. Amorati, L. Valgimigli, Modulation of the antioxidant activity of phenols by non-Covalent Interactions, Org. Biomol. Chem. 10 (2012) 4147–4158.
- P.-G. Pietta, Flavonoids as antioxidants, J. Nat. Prod. 63 (2000) 1035–1042, https://doi.org/10.1021/np9904509.
- J.Z. Xu, S.Y.V. Yeung, Q. Chang, Y. Huang, Z.-Y. Chen, Comparison of antioxidant activity and Bioavailability of Tea Epicatechins with their epimers, Br. J. Nutr. 91 (2004) 873–881, https://doi.org/10.1079/BJN20041132.
- A.B. Enogieru, W. Haylett, D.C. Hiss, S. Bardien, O.E. Ekpo, Rutin as a potent antioxidant: Implications for Neurodegenerative disorders, Oxid. Med. Cell. Longev. 2018 (2018) 6241017, https://doi.org/10.1155/2018/6241017.
- G.S.B. Aseervatham, U. Suryakala, S. Sundaram, P.C. Bose, T. Sivasudha, Expression pattern of NMDA receptors reveals antiepileptic potential of apigenin 8-C- glucoside and chlorogenic acid in pilocarpine induced epileptic mice, Biomed. Pharmacother. 82 (2016) 54–64.
- Y. Wang, Y. Zhen, X. Wu, Q. Jiang, X. Li, Z. Chen, et al., Vitexin protects brain against ischemia/reperfusion injury via modulating mitogen-activated protein
kinase and apoptosis signaling in mice, Phytomedicine 22 (2015) 379–384, https://doi.org/10.1016/j.phymed.2015.01.009.
- I. Guti´errez-Del-Río, S. Lo´pez-Ib´an˜ez, P. Magada´n-Corpas, L. Ferna´ndez-Calleja, A´. P´erez-Valero, M. Tun˜o´n-Granda, et al., Terpenoids and polyphenols as
natural antioxidant agents in food preservation, Antioxid. Basel Switz. 10 (2021) 1264, https://doi.org/10.3390/antiox10081264.
- J.E. Krajicek, R.D. Williams, Celtisoccidentalis L. Hackberry, Silv. N Am. 2 (1990) 262–265.
- M.R. Gilmore, Uses of plants by the Indians of the Missouri river region, Available, http://repository.si.edu/xmlui/handle/10088/91746, 1919. (Accessed 7 September 2023).
- G. Usher, A Dictionary of Plants Used by Man, Constable, London, 1974.
- O. Polunin, Flowers of Europe. A Field Guide, Flowers Eur Field Guide, 1969.
- J.S. Gamble, A Manual of Indian Timbers, РиполКлассик, 1881.
- R.A. Vines, Trees of Central Texas, University of Texas Press, 1984.
- C.S. Sargent, Manual of the Trees of N. America, Vol. I & II, Dover Publications Inc., New York, 1965.
- R.A. Vines, Trees of North Texas, University of Texas Press, 1982.