Cytotoxic, Analgesic, and Antidiarrheal Activities from the Seeds of Commonly Available Red Grape (Vitis vinifera L.)
ABSTRACT
OBJECTIVES: The current study aimed to conduct a phytochemical screening of commonly known fruit red grape (Vitis vinifera L.) seed methanolic extract through gas chromatography and mass spectrometry (GC-MS) to identify the bioactive compounds responsible for its health benefits and evaluate the pharmacological potentialities of the extract and its fractions against oxidation, inflammation, pain, and diarrhea.
METHODS: The in vitro antioxidant, anti-inflammatory, and cytotoxic characteristics of methanolic extracts and various solvent fractions of V. vinifera were evaluated using the DPPH free radical scavenging assay, membrane stabilizing, and brine shrimp lethality bioassay. Furthermore, the study assessed the effects of crude extracts (200, 400, and 600 mg/kg of body weight) on pain relief and reduction of diarrhea in animals using methods such as tail immersion, the acetic acid-induced writhing technique, and a diarrheal mouse model induced with castor oil.
RESULTS: A total of 73 phytoconstituents were predominantly found in the seed extract based on the GC-MS analysis. Among the identified compounds, 9-octadecenamide (13.7%), and (9E,11E )-octadeca-9,11-dienoate (11.07%) are most abundant. Several notable constituents, such as gamma-sitosterol, stigmasterol, paromomycin, 4,6-cholestadienol, gamma-tocotrienol, 24-Propylidenecholest-5-en-3beta-ol, and alpha-tocopherol acetate, are also present. The methanolic extract of V. vinifera seed and its different solvent fractions showed promising anti- oxidant properties (IC50 = 1.19-17.42 µg/mL) compared to the standard antioxidant butylated hydroxytoluene (IC50 = 20.46 µg/mL). Aqueous soluble fraction exerted inhibition of nearly 50% heat-induced hemolysis compared to the standard acetylsalicylic acid (42%). Besides, all the tested doses (200, 400, and 600 mg/kg bw) of the crude extract showed significant (P < .05) analgesic and antidiarrheal effects.
CONCLUSION: The current findings endorsed the health benefits of V. vinifera by revealing potent antioxidant, anti-inflammatory, analgesic, and antidiarrheal effects. Nevertheless, further in-depth analysis of the plant’s chemical constituents and pharmacological effects on health is warranted for novel drug discovery from V. vinifera.
KEYWORDS: Red grape seed, GC-MS analysis, antioxidant, anti-inflammatory, cytotoxicity, analgesic, antidiarrheal
RECEIVED: October 20, 2023. ACCEPTED: July 17, 2024.
TYPE: Original Research
Introduction
Fruit is classified as a botanical product originating from the reproductive structures of plants, constituting a vital component of the human and animal diet. Fruits serve as a primary energy source while offering essential nutrients and an array of bioactive compounds crucial for maintaining optimal health. Furthermore, fruits exhibit health-promoting properties, mitigating susceptibility to particular ailments and age-related functional degenerations.1 A significant correlation between a diet rich in antioxidants and diminished susceptibility to chronic illnesses was established in various research.2 Cereals, legumes, oilseeds, as well as fruits and vegetables, serve as primary sources of dietary polyphenols and numerous bioactive constituents.3,4
These components play pivotal roles in enhancing the functional and nutraceutical qualities of the diet.3 Moreover, it is noteworthy that not only the consumable portions of fruits but also the residual byproducts amount contain substantial well-known for of phenolic compounds and bioactive phytochemicals. These compounds are their notable antioxidant, anti-inflammatory, anti-mutagenic, and anti-carcinogenic properties.3,5,6
Food loss and waste have emerged worldwide, manifesting at different points along the food supply chain. In 2019, hun- ger or insufficient access to nutritious food affected around 2 billion people, equivalent to about a quarter (25.9%) of the global population. Conversely, each year, about one-third (1.3
billion metric tons) of the food produced for human con- sumption goes to waste throughout the food supply chain.7 This wastage also entails substantial nutritional losses, with foods rich in nutrients like fruits and vegetables being the most commonly discarded. Consequently, reducing or reallocating food waste has the potential to enhance food accessibility and simultaneously elevate nutritional and dietary standards.
The most common fruits consumed globally encompass apples, grapes, and exotic fruits indigenous to their respective cultivation regions. Grapes notably constitute the largest fruit crop worldwide, boasting an annual production exceeding 75 million tons globally.8 Within this framework, grapes and their derivatives, including wine, grape juice, and preserves, have significant economic importance and exert a substantial influence on waste generation. Residues such as marc, peels, and grape seeds, persist due to inadequate management practices, thereby posing a contamination risk.8 Conversely, grape byproducts serve as a valuable reservoir of essential nutrients, encompassing vitamins, minerals, lipids, proteins, carbohydrates, and polyphenolic compounds.6
The red grape (Vitis vinifera L.; Family: Vitaceae), which grows in the Mediterranean and Central Asia, is a commonly consumed fruit rich in nutrients, which offers many health advantages.9 However, the winemaking sector is renowned for producing significant quantities of byproducts, and managing their disposal presents economic and environmental challenges. This issue of food waste has drawn considerable public health concern. Hence, there is value in conducting research to trans- form food waste into a usable resource and tackle worldwide nutritional deficiencies.10,11
In several experimental investigations, red grape (Vitis vinifera L.) seed extract, which is a byproduct of manufacturing wine and is high in pro-anthocyanidins, has demonstrated promise. Research indicating its effectiveness against hyper- tension, inflammation, peptic ulcers, microbiological infections, cardiovascular disease, cancer, and diabetes has confirmed its pharmacological activity and beneficial health effects. Extracts of grape seeds are poised to be turned into a potential therapeutic due to their wide variety of applications.12 In animal experiments, grape seed extract has demonstrated preventive properties, including suppression of DNA breakage, lipid peroxidation, and reduction of cardiac infarct size. It has been shown to inhibit lineages of human cancer cells in vitro.13
The current investigation utilizes GC-MS analysis to identify bioactive components from the methanolic extract of red grape (V. vinifera) seeds. This research additionally explored the pharmacological potentialities of the fruit seeds against oxidation, inflammation, pain, and diarrhea. These outcomes of the research may be helpful in spreading the nutraceuticals and medicinal value of the V. vinifera seeds, which is commonly available in Bangladeshi market.
Methods and Materials
Collection of V. vinifera seeds and drying
The Vitis vinifera (Family: Vitaceae), locally known as red grapes, were purchased from the local market of West Dhanmondi, Dhaka, Bangladesh, in January 2022. The batch was collected from Yantai, Shandong province, China. Following the acquisition of the fruit from the market, the process entailed the manual collection of fresh seeds, which were subsequently subjected to washing with fresh water. The seeds underwent a drying phase through natural shedding, facilitated by exposure to open air for an extended period. During this drying regimen, stringent measures were implemented to cosistently maintain an environmental temperature below 30°C. This precautionary measure was adopted to ensure the preservation of heat-sensitive compounds and to preempt any possibility of degradation. Supplemental Figure S1 illustrates the dried seeds of V. vinifera.
Chemicals and reagents
Reagents of Analytical-level and substances were utilized in this investigation. All the chemicals such as Tween 80, tert- butyl-1-hydroxytoluene (BHT), Folin Ciocalteau reagent, and Gallic acid were collected through Merck (Germany). Sigma- Aldrich (USA) supplied 2,2-diphenyl-1-picrylhydrazyl (DPPH). BEXIMCO Pharmaceuticals Ltd., Dhaka, Bangladesh, delivered the glibenclamide, normal saline solution, and loperamide as gift samples.
Extraction
A high-capacity grinding apparatus was employed to trans- form the desiccated seeds into a coarse powder. The extraction process followed the solid-liquid extraction method. It is noted that 500 g of powdered seeds were placed inside a sanitized amber bottle with a 3.0 L capacity. Subsequently, the powder was immersed in 2.0 L of methanol and allowed to soak for a period of 15 days at room temperature, with intermittent shak- ing and stirring. After that, a fresh cotton pad and filter paper were used to filter the solvent mixture. In order to produce a concentrated crude extract, the methanol was subsequently separated using an “EYELA Rotavapor” rotary evaporator at smaller pressures and about 40 to 50°C. Ultimately, a quantity of 67 g of gummy exudate was acquired from V. vinifera, signifying a yielding rate of 13.4%.
Fractionation
For the solvent-solvent differentiation of the extract, S. Morris Kupchan’s partitions (1970) technique,modified by VanWagenenet et al14 as used. It was accomplished by extracting 5g of crude extract with water in methanol (1:9), petroleum ether (PESF), dichloromethane (DCMSF), ethyl acetate (EASF), and water
(AQSF) to produce 4 distinct fractions. Each fraction also continued to evaporate until it was completely dried. The yields for the fractions produced by petroleum ether, dichloromethane, ethyl acetate, and water solubility were 25%, 24%, 18%, and 27%, respectively.
Gas Chromatography Mass Spectrometry (GC- MS) analysis
With an auto-sampler, Shimadzu, Japan, GC/MS-QP2010 ultra was used to analyze the phytoconstituents of the V. vinifera seeds’ methanolic extract. In a 5 MS/HP column (30 m, 0.25 mm, and 0.25 m), extremely pure helium was used as the mobile phase. The linear speed of the helium was 39 cm/s, and the helium circulation rate was 1.12 mL/min. The oven temperature was kept constant at an average rate of 10°C per minute, ranging from 110°C to 280°C. The needle temperature was adjusted to 250°C. The injection volume (50 µL) was entered in splitless mode (ratio of 10:1). The detector voltage was maintained at 0.94 kV. The ambient temperatures of the ion source, MS transfer line, and the ion source were all kept at 200 and 250°C, respectively. Full-scan mass spectra with a (m/z) range of 85 to 500 were captured at 10 000 u/s. Searching in the National Institute of Standards and Technology (NIST) collection yielded to identify peaks and chemical constituents.
Antioxidant properties
Total phenolic content (TPC). The TPC of the seeds’ methanolic extract and fractions was determined using the Folin Ciocalteu method.15 One milliliter of each fraction and extract (2 mg/mL) was combined with Na2CO3 (2.5 mL, 7.5% w/v) and a 10-fold diluted Folin Ciocalteu reagent (2.5 mL). The solution was incubated in the dark at room temperature for 30 minutes. A UV-Vis spectrophotometer (760 nm) was used to test the sample’s absorbance. The calibration standard curve (Gallic acid) was created by placing the absorbance against various Gallic acid concentrations (100, 50, 25, 12.5, 6.25, 3.12, 1.56, 0.78, and 0.39 µg/mL) to calculate the TPC of the samples stated on the label as GAE mg (Gallic acid equivalent)/g of the study’s samples. The Gallic acid calibration curve formulations are as follows:
y = 0.0162x + 0.0215, R2 = 0.9985
DPPH scavenging assay. Antioxidant activity was qualitatively and quantitatively assessed using thin-layer chromatography (TLC) and the stable free radical 0.04% DPPH (1,1-diphenyl- 2-picrylhydrazyl).16 To separate the polar and non-polar components of the extract, diluted stock solutions were applied to a dyed silica gel TLC plate, which was then placed in chambers containing solvents of varying polarities (polar, medium polar, and non-polar). The plate was subsequently sprayed with
0.02% DPPH in ethanol and incubated at room temperature. Following the DPPH treatment, color shifts (yellow on a purple background) were observed on the resolved bands for 10 minutes.16
The DPPH approach was employed to assess the antioxidant potential of MECE and various fractions.17 Initially,
3.0 mL of freshly made methanol and DPPH solution (20 µg/ mL) was thoroughly combined with 2 mL of the samples’ various strengths (500, 250, 125, 62.5, 31.25, 15.62, 7.81, 3.9, 1.93,
and 0.97 µg/mL) before being placed in a dark environment at room temperature for 30 minutes. The samples’ absorbance was then measured with a UV-Vis spectrophotometer calibrated to 517 nm. The inhibition percentage (I%) of the DPPH free radical was calculated using the following formula17:
I% = (Acontrol – Asample )/Acontrol * 1OO
Acontrol indicated the absorbance of the solution that had all reagents but lacked the test components. Asample indicated the absorbance of the samples being examined or the standard (BHT) solution. Every test sample generated a graph showing the percentage of inhibition versus the concentrations of the tested substances, and the IC50 (half concentration of the greatest inhibitory concentration) values were then calculated using a linear regression approach.
Anti-inflammatory effects
The effectiveness of V. vinifera seed extract and fractions to stabilize human erythrocyte membranes was investigated using hypotonic and heat-induced techniques.18,19
Hypotonic solution-induced hemolysis. Red blood cells (RBCs) were given to a 70 kg adult with fair skin and no hidden disorders. The RBCs were put in a sterile container containing an anticoagulant, EDTA. A buffer solution with a pH of 7.4 was created using disodium phosphate and its conjugate acid, monosodium phosphate. 4.5045 g of NaCl was dissolved in sterile distilled water to make an isotonic solution (500 mL, 154 mM). 1.4625 g of NaCl was dissolved in sterile distilled water to make a hypotonic solution (500 mL, 50 mM). The blood was cleaned 3 times with sodium phosphate buffer and isotonic solution before centrifugation (3000 rpm, 10 minutes) to get the erythrocyte suspension. A stock RBC suspension (0.50 mL), a buffer of 10 mM sodium phosphate (4.5 mL), and a hypo- tonic solution (50 mM NaCl) were used as test samples, with different proportions of MECE (2.0 mg/mL) or standard ASA (0.10 mg/mL) as the reference standard. Before centrifugation (3000 rpm, 10 minutes), the resulting solutions were incubated at ambient temperature for 10 minutes. The supernatant’s absorbance (optical density (OD)) was then measured at 540 nm. The following calculation was used to compute the hemolysis inhibition percentage or membrane stabilization of the tested samples and standard ASA:
% Hemolysis inhibition
(hypotonic solution induced )
=100 *(OD1 -OD2 ) /OD3
The mice were kept in polypropylene cages under standard climatic settings, including a 25°C ambient temperature, a 55°% relative humidity level, and a 12-hour light/dark cycle before the
OD1: hypotonic buffered saline solution optical density
(Control)
OD2: optical density of the examined sample in hypotonic solution
Heat-induced hemolysis. Two batches of centrifuge tubes containing 5 mL of isotonic buffer and 1.0 mg/mL of seed extract and various fractions were made. One tube with the same amount of material was used as a control. Each tube was filled with 30 µL of erythrocyte suspension and stirred slowly by inversion. A single set of tubes was submerged in a 54°C water bath for 20 minutes, whereas the additional set was maintained in a 0 to 5°C cold bath. Following incubation, the supernatant was turned to a centrifuge (1300 rpm, 3 minutes) and the optical density (OD) was measured at 540 nm. The percentage inhibition of hemolysis in the study was estimated using the following equation:
animal study began. During this time, ICDDRB prepared food and water for them to consume. The study followed the rules established by the Federation of European Laboratory Animal Science Associations (FELASA) for the moral treatment of animals in investigations. The Animal Ethics Committee of State University of Bangladesh also carefully examined and confirmed the research’s ethical standards and procedures. In every test, mice were divided into 5 distinct groups; negative control, positive control, and 3 different (200, 400, and 600 mg/kg bw) sample groups I-III. Every group had 2 male and 2 female mice. Since we could not determine the standard deviation and effect size, we employed the “resource equation” method outlined by Mukta et al5 and Bulbul et al20 to estimate the sample size. While working with a 1-way analysis of variance (ANOVA), we calculated the sample size for the degrees of freedom associated with the variability between subjects, also known as within-subject degrees of freedom, using the formula:
% hemolysis
æ1- OD2 -OD1 / ö
n = DF/k + 1
inhibition (heat-induced )
=100 *ç çOD3-OD1 ÷
Here, OD1 = optical density (OD) of the test sample unheated, OD2 = OD of test sample heated, and OD3 = OD of control sample heated.
Cytotoxicity: brine shrimp lethality bioassay
The methanolic extract and its fractions of V. vinifera seeds were assessed for their cytotoxic effects through a brine shrimp lethality test described by Rashid et al,19 utilizing vincristine sulfate (VS) as a positive control. To create varying concentrations (ranging from 400.0 to 0.781 µg/mL) of the samples, a serial dilution of each 4 mg test sample was prepared in 99% DMSO (dimethyl sulfoxide). These solutions were then exposed to simulated seawater containing around 10 live brine shrimp nauplii. After a 24-hour period, the surviving nauplii were scrutinized using a magnifying glass. The toxicity level of the shrimp in response to each concentration of the sample was determined to estimate the LC50 value. The LC50 value, representing the concentration at which 50% of the shrimp were not viable, was calculated from a graph plotting the percentage of non-viable shrimp against the logarithmic concentration of the plant extract, utilizing the vincristine standard curve as reference.
Experimental design and sample size determination for in vivo studies
Swiss albino adult mice that were healthy, weighed between 25 and 30 g, and were between 4 and 5 weeks’ old were collected from the Animal Division of the International Center for Diarrheal Diseases and Research in Bangladesh (ICDDRB).
In this formula, “n” represents the number of animals in each group, “DF” signifies the degrees of freedom, and “k” denotes the total number of groups. We considered the allowable range for degrees of freedom (DF) to establish the minimum and maximum number of animals per group. We obtained the respective minimum and maximum numbers of animals per group by using the minimum (10) and maximum (20) values for DF. In this case, we excluded the normal control group by setting ’k’ to 4, indicating 4 groups (comprising 3 test groups I-III with doses of 200, 400, and 600 mg/kg bw, and 1 positive control group with the standard drug). Therefore, the mini- mum and maximum numbers of animals per group should be 4 (10/k + 1 = 10/4 + 1) and 6 (20/k + 1 = 20/4 + 1), respectively. Following these ethical principles, we carried out this preliminary investigation with the minimal number of animals per group (n = 4). At the end of the study, according to the AVMA (American Veterinary Medical Association) guidelines for the Euthanasia of Animals, all mice were compassionately euthanized while under general anesthesia.20
Central analgesic activity
The central analgesic effects of the MECE were evaluated using a heat approach and the tail immersion experiment.21,22 The given morphine (15 mg/mL) was diluted with saline water to form the standard sample (subcutaneous, 2 mg/kg). The test medications were orally administered to the mice using a feeding syringe. The test included submerging a mouse’s tail in water heated to 55°C. At 0, 30, 60, and 90 minutes after giving the test samples to each mouse, the PRT or latency duration to move its tail in hot water was assessed.
Peripheral analgesic activity
The acetic acid-induced writhing method was employed to assess the peripheral analgesic effects of MECE.22 Each animal group received glacial acetic acid, a substance that induces pain. Tween 80 was used as a negative control, Diclofenac sodium (50 mg/kg) as a positive control, and MECE (200, 400, and 600 mg/kg) were given to the distinctive mouse groups. Acetic acid is in charge of writhing. The number of writhes was counted after delivering acetic acid intraperitoneally for 10 minutes. The following percentage of writhing inhibition was calculated:
group. The average values with their associated standard errors of the mean was expressed as mean ± SEM to summarize the in vivo results. To analyze the in vivo data, we used Statistical Package for Social Sciences (SPSS) Software (version 26.0), conducting a 1-way analysis of variance (ANOVA) on all variables, followed by a student’s t-test. Any P-values below .05 were considered statistically significant.
Results
Identification of phytoconstituents by GC-MS analysis
Writhing inhibition % =(NControl – Ntest ) /NControl ´100%
A total of 73 phytoconstituents were predominantly found in the MECE based on their GC-MS analysis. The spectrum
where N = average amount of stomach writhing for every group.
Anti-diarrheal activity
A castor oil-induced diarrhea model in mice was implemented to evaluate the anti-diarrhea activity of MECE, as stated by earlier investigation.23 Each of the 3 groups—the control, positive control, and test groups—contained 4 mice each. The control group received 1% Tween 80 in normal saline as a carrier solution and only orally at a specific dose (10 mL/kg). While different doses (200, 400, and 600 mg/kg) of MECE were pro- vided to the 3 test groups with Tween 80 (1% in normal saline) as a carrier, loperamide (50 mg/kg) was administered orally to the positive control group. Each mouse was administered 1 mL of pure castor oil to induce diarrhea after 1 hour. Each mouse was housed in an individual cage placed on the floor, lined with blotting paper. The quantity of diarrheal stools for each mouse was recorded at the conclusion of every hour for up to 4 hours following the administration of castor oil. The blotting paper was replaced at the beginning of each hour. Observations from the test groups were compared with those from the negative and positive control groups to evaluate the potential of MECE as an antidiarrheal agent. The percentage reduction in diarrhea was calculated using the following formula to assess the antidiarrheal activity.23
% inhibition of defecation = (Dcontrol – Dtest ) /Dcontrol ´100%
where D = Average diarrheal episode/number in every group.
Statistical analysis
MS Excel (version 10.0) was used for processing the graphs and data related to in vitro investigation. Because the in vitro data was derived from a single experiment, there was no statistical analysis conducted for comparing between groups; instead, a numerical comparison was carried out. Instead, statistical analysis was done on the data collected from the in vivo tests. We compared the treatment groups to the control (vehicle)
obtained from GC-MS analysis is available as Figure 1. Among the isolated compounds, 9-Octadecenamide (13.7%), (9E,11E)-octadeca-9,11-dienoate (11.07%), 1,3-dihydroxy-
propan-2-yl(9Z,12Z)-octadeca-9,12-dienoate (9.06%), and Tris(hydroxymethyl)nitromethane (7.93%) were most abundant (Table 1), while squalene (0.08%), 13-Docosenoicacid, methyl ester (Z)- (0.1%), and Vitamin E (0.1%) were the least amount compounds (Table 1). Some bioactive plant steroids such as gamma-sitosterol, stigmasterol, Ergost-5-en-3-ol, (3beta, 24R)-, and 24-Propylidenecholest 5-en-3beta-ol were also detected from the MECE. In addition, some notable com- pounds are paromomycin, 4,6-cholestadienol, gamma-tocot-rienol and alpha-tocopherol acetate. According to GC-MS analysis, all identified compounds’ names, retention time (RT),
% area, molecular formula, molecular weight, PubChem CIDs, and chemical structures were tabulated in Table 1. In addition, the reported bioactivities of these identified compounds were also tabulated with their corresponding references (Table 1).
Assessment of antioxidant properties
Total phenolic content (TPC). The findings revealed that the MECE expressed the most TPC (223.31 mg of GAE/g of dried extract), then the EASF and DCMSF fractions at
190.25 mg of GAE/g and 135.25 mg of GAE/g, respectively. In contrast, the PESF fraction indicated the least TPC, with
11.00 mg of GAE/g (Figure 2). These results suggest that the phenolic profiles of the various fractions of the seed extract are diverse, which may have consequences for their prospective bioactivity and use.
DPPH scavenging activity. In this study, the antioxidant potency of methanolic extract of V. vinifera seed and its different solvent fractions showed promising antioxidant properties (IC50 = 1.19-17.42 µg/mL) compared to that of the standard antioxidant butylated hydroxytoluene (BHT; IC50 = 20.46 µg/ mL; Figure 3). The EASF has the highest free radical scavenging potency (IC50 = 1.19 µg/mL), followed by MECE (IC50 = 2.94 µg/mL), AQSF (IC50 = 6.55 µg/mL), PESF (IC50 = 16.81 µg/mL), and DCMSF (IC50 = 17.42 µg/mL).
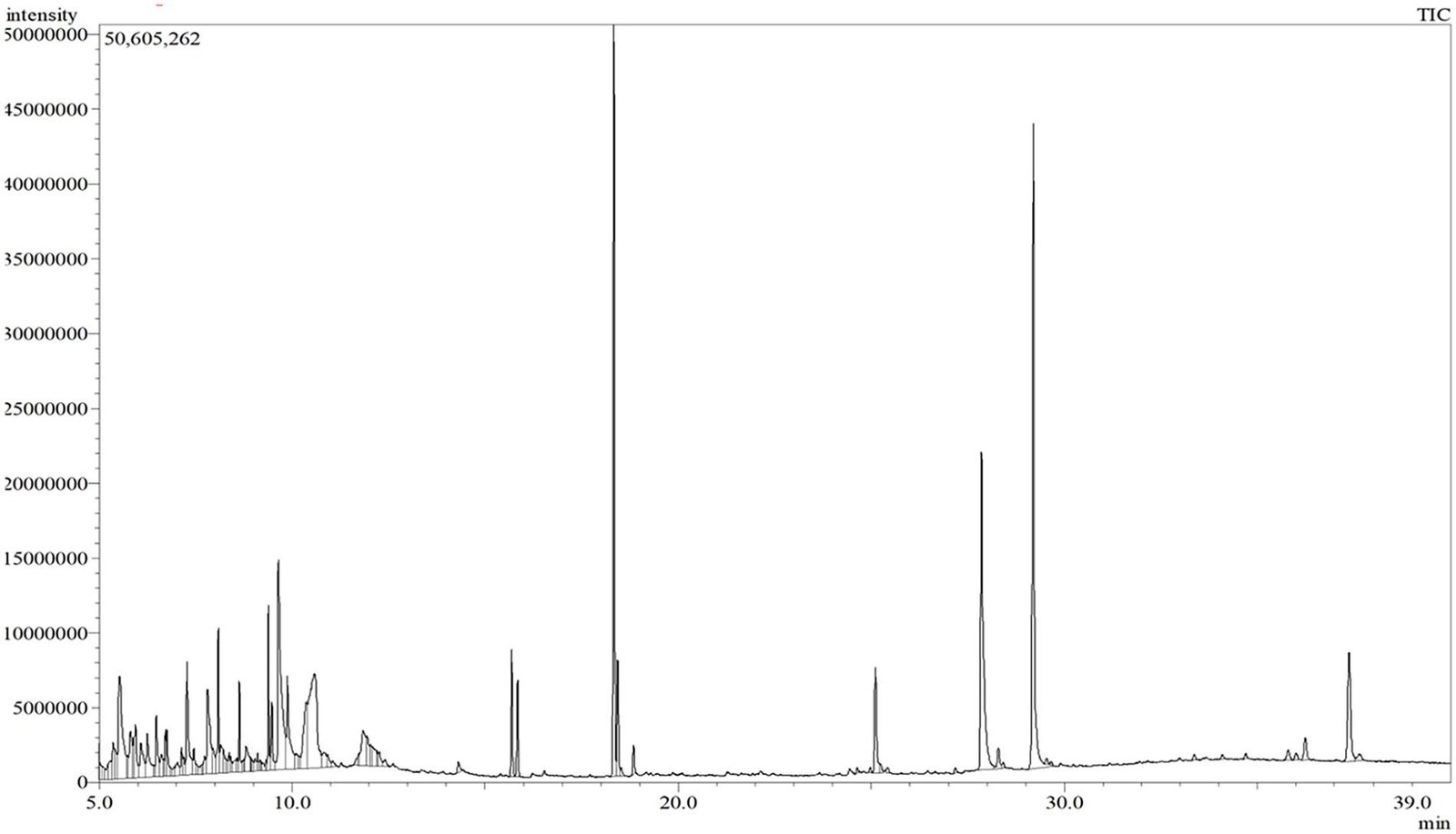
Figure 1. GC-MS spectrum of the crude extract obtained from V. vinifera L.
Anti-inflammatory effects
Hypnotic medium-induced hemolysis has been diminished as below: AQSF (22.62%), DCMSF (13.62%), PESF (10.39%),
MESF (6.53%), and EASF (7.10%). The standard acetylsalicylic acid (ASA) inhibition rate was significant (61.90%; Figure 4). The most efficacious inhibitor of heat-induced hemolysis was AQSF (44.9%), followed by DCMSF (31.15%), PESF (21.65%), MESF (13.43%), and EASF (13.50%). The
inhibition of the standard ASA was 42.00% (Figure 4).
Cytotoxicity
Each sample that underwent testing displayed noteworthy lethality against brine shrimp larvae, as evidenced by their LC50 values (15.95-83.24 µg/mL), which were comparable to the established benchmark set by vincristine sulfate with an LC50 of 0.45 µg/mL (Figure 5).
Central analgesic activity
As stated in Figure 6, all the doses of the tested sample expressed significant (P < .05) dose- and time-dependent analgesic properties compared to standard morphine. The groups of ingesting MECE at various doses demonstrated a rise in pain reaction latency compared to the control, whereas the negative control possessed no analgesic impact.
Peripheral analgesic activity
The study found that MECE showed noticeable and dose- dependent activity in peripheral analgesic assessment com- pared to the negative control group.The outcomes demonstrated
that the seeds extracted from groups I to II exhibited a time- and dose-dependent reduction in writhing activity in mice (Figure 7). The seeds extract at group II and group III exerted 36.78% and 43.68% writhing inhibition in mice, around half of the standard diclofenac sodium (77.01% inhibition; Supplemental Figure S2). All the sequels were statistically significant with P < .05, indicating that the MECE might have a peripheral analgesic effect in mice in response to acetic acid- induced stomach writhing.
Anti-diarrheal activity
The results of the MECE indicated a statistically significant reduction in the total amount of diarrheal feces at all doses of MECE (Figure 8). Notably, the 400 mg/kg dose of MECE demonstrated the topmost degree of activity (80.43%) com- pared to the positive control Loperamide (78.26%) for the reduction of diarrhea (Supplemental Figure S3).
Discussion
Herbal remedies, including parts like fruit, seeds, bark, fruit peel, and leaves, are frequently viewed as valuable sources of bio- active phytochemicals for addressing various health issues such as oxidative stress, diabetes, pain, fever, cancer, hypertension, and many more human illness.77 The present research examined the chemical components within the seed extract of V. vinifera. This analysis revealed a range of potentially valuable bioactive elements. These elements could play a crucial role in driving the diverse bioactive effects observed in the red grape seed extract and its different solvent-based fractions. The GC-MS analysis of the MECE expressed the presence of several phytoconstituents, such as organic acids (pentanedioic acid, 2-amino octanoic
Hossain et al
7
|
SL. NO. |
COMPOUND |
RT |
AREA % |
MOL. WEIGHT (G/MOL) |
MOLECULAR FORMULA |
PUBCHEM CID |
CHEMICAL STRUCTURES |
BIOLOGICAL ACTIVITY |
REF |
|
1 |
Pentanedioic acid |
5.027 |
0.39 |
132.11 |
C5H8O4 |
743 |
Anti-neoplastic activity |
Goswami et al24 |
|
|
2 |
Decyl tetradecyl ester carbonic acid |
5.105 |
0.32 |
272.423 |
C25H50O3 |
91693142 |
Antioxidant, Antibacterial activity |
Gopal Pandit et al25 |
|
|
3 |
2,4-dihydroxy-2,5- dimethylfuran-3(2H)-one |
5.365 |
0.82 |
144.12 |
C6H8O4 |
538757 |
|
Cerny26 |
|
|
4 |
Tranylcypromine |
5.41 |
0.78 |
133.19 |
C9H11N |
5530 |
MAO inhibitor
|
Tranylcypromine27 |
|
|
5 |
1-(furan-2-yl)-2-methylprop- 2-en-1-one |
5.532 |
4.56 |
136.15 |
C8H8O2 |
3007408 |
Anti-fungal activity
|
Ogata et al28 |
|
|
7 |
Limonene |
5.817 |
1.46 |
136.23 |
C10H16 |
22311 |
Anti-bacterial activity
|
Han et al29 |
|
(Continued)
Nutrition and Metabolic Insights
8
|
SL. NO. |
COMPOUND |
RT |
AREA % |
MOL. WEIGHT (G/MOL) |
MOLECULAR FORMULA |
PUBCHEM CID |
CHEMICAL STRUCTURES |
BIOLOGICAL ACTIVITY |
REF |
|
|
8 |
Maple lactone |
5.91 |
0.55 |
112.12 |
C6H8O2 |
6660 |
|
Anti-oxidant activity |
Kim et al30 |
|
|
10 |
2-amino octanoic acid |
6.09 |
1.36 |
159.22 |
C8H17NO2 |
69522 |
H2N |
OH O |
Antibiotic activity |
Almahboub et al31 |
|
11 |
Furaneol |
6.251 |
1.46 |
128.13 |
C6H8O3 |
19309 |
|
|
Anti-microbial & Anti-fungal activity |
Sung et al32 |
|
12 |
1,3-Propanediol, 2-methyl- 2-propyl |
6.435 |
0.26 |
370.38 |
C11H22N4O8S |
117593
|
|
Sedative, anticonvulsant and muscle relaxant effect
|
Yale et al33 |
|
|
13 |
Thymine |
6.489 |
1.19 |
126.11 |
C5H6N2O2 |
1135 |
|
|
Anti-cancer & Anti-microbial activity
|
Kumar et al34 |
(Continued)
Hossain et al
9
|
SL. NO. |
COMPOUND |
RT |
AREA % |
MOL. WEIGHT (G/MOL) |
MOLECULAR FORMULA |
PUBCHEM CID |
CHEMICAL STRUCTURES |
BIOLOGICAL ACTIVITY |
REF |
|
14 |
2-methoxy phenol |
6.617 |
0.6 |
124.13 |
C7H8O2 |
460 |
Antibacterial and antioxidant activity
|
Rubab et al35 |
|
|
15 |
Nonane,5-(1-methylpropyl) |
6.683 |
0.21 |
184.36 |
C13H28 |
43943 |
Antioxidant and Antimicrobial activity |
Ashraf et al36 |
|
|
16 |
Diazene, bis(1,1- dimethylethyl)- |
6.725 |
0.61 |
142.24 |
C8H18N2 |
70227 |
Anti-phytopathogenic
|
Singh et al37 |
|
|
19 |
1,2,4-Benzenetriol |
6.94 |
0.22 |
126.11 |
C6H6O3 |
10787 |
|
Zhang et al38 |
|
|
21 |
2-Propanamine, N-methyl- N-nitroso |
7.141 |
0.48 |
102.14 |
C4H10N2O |
92271 |
Anti-bacterial
|
Kadiri et al39 |
|
(Continued)
Nutrition and Metabolic Insights
10
|
SL. NO. |
COMPOUND |
RT |
AREA % |
MOL. WEIGHT (G/MOL) |
MOLECULAR FORMULA |
PUBCHEM CID |
CHEMICAL STRUCTURES |
BIOLOGICAL ACTIVITY |
REF |
|
22 |
Limonene oxide |
7.197 |
0.3 |
152.23 |
C10H16O |
8029780 |
Bactericidal, anticancer,
|
Junior and Pastore40 |
|
|
23 |
2,3-Dihydro-3,5-dihydroxy- 6-methyl-4h-pyran-4-one |
7.282 |
2.56 |
144.12 |
C6H8O4 |
119838 |
Anti-oxidant and mutagenic
|
41 |
|
|
24 |
4 chloroanisole |
7.459 |
0.49 |
142.583 |
C7H7ClO |
11137567 |
Antibacterial activity
|
Boubakri et al42 |
|
|
25 |
5-methyl-4H-1,2,4-triazol-3- amine |
7.545 |
0.2 |
98.11 |
C3H6N4 |
234610 |
Antiviral & Anti-infective activity
|
43 |
|
|
28 |
Catechol |
7.81 |
2.88 |
110.11 |
C6H6O2 |
289 |
Anti-microbial, Anti-fungal activity
|
Kocaçalışkan et al44 |
|
(Continued)
Hossain et al
11
|
SL. NO. |
COMPOUND |
RT |
AREA % |
MOL. WEIGHT (G/MOL) |
MOLECULAR FORMULA |
PUBCHEM CID |
CHEMICAL STRUCTURES |
BIOLOGICAL ACTIVITY |
REF |
|
29 |
2-methyle-5(1-methylethyl)- cyclohexanone |
7.957 |
0.63 |
154.25 |
C10H18O |
10362 |
Weak fungicidal activity
|
Xia et al45 |
|
|
30 |
(1R,5R)−2-methyl-5-(prop- 1-en-2-yl)cyclohex-2-enol |
8.09 |
1.86 |
152.23 |
C10H16O |
11084068 |
Anti-bacterial & Anti-fungal activity
|
Aberchane et al46 |
|
|
31 |
5-(hydroxymethyl) furan-2-carbaldehyde |
8.152 |
0.86 |
126.11 |
C6H6O3 |
237332 |
Anti-oxidant & Anti- proliferative
|
Qiu et al47 |
|
|
32 |
Carveol |
8.23 |
0.36 |
152.23 |
C10H16O |
7438 |
Anti-oxidant, Anti-cancer & vasorelaxation
|
Lacerda-Neto et al48 |
|
|
35 |
cis-Limonene oxide |
8.638 |
1.05 |
152.23 |
C10H16O |
6452061 |
Anti-microbial & insecticidal activity
|
He et al49, Aggarwal et al50 |
|
(Continued)
Nutrition and Metabolic Insights
12
|
SL. NO. |
COMPOUND |
RT |
AREA % |
MOL. WEIGHT (G/MOL) |
MOLECULAR FORMULA |
PUBCHEM CID |
CHEMICAL STRUCTURES |
BIOLOGICAL ACTIVITY |
REF |
|
36 |
4-Methyl catechol |
8.807 |
0.88 |
C7H8O2 |
9958 |
|
Ito et al51 |
||
|
37 |
o-Tolunitrile |
8.91 |
0.13 |
117.15 |
C8H7N |
10721 |
NA
|
||
|
38 |
Cyclopropane |
8.94 |
0.16 |
42.08 |
C3H6 |
6351 |
NA |
||
|
39 |
Decyl 2-chloroacetate |
8.975 |
0.18 |
234.76 |
C12H23ClO2 |
229381 |
|||
|
40 |
2-Methoxy 4-vinylphenol |
9.046 |
0.29 |
150.17 |
C9H10O2 |
332 |
Antimicrobial, antioxidant, anti-inflammatory, analgesic, anti-germination
|
Ibibia et al52 |
|
|
41 |
3-(Methylthio)propyl acetate |
9.165 |
0.13 |
148.23 |
C6H12O2S |
85519 |
Flavor activity |
Aoki and Uchida53 |
|
|
43 |
1-Methoxy-6,6- dimethylcyclohex-1-ene |
9.24 |
0.18 |
140.22 |
C9H16O |
580527 |
NA
|
||
(Continued)
Hossain et al
13
|
SL. NO. |
COMPOUND |
RT |
AREA % |
MOL. WEIGHT (G/MOL) |
MOLECULAR FORMULA |
PUBCHEM CID |
CHEMICAL STRUCTURES |
BIOLOGICAL ACTIVITY |
REF |
|
44 |
9,12,15-Octadecatrienoic acid, methyl ester, (9Z,12Z,15Z)- |
9.329 |
0.15 |
292.5 |
C19H32O2 |
9316 |
Antioxidant, anti- carcinogenic, anti- inflammatory, and anti-obese |
Yuan et al54 |
|
|
45 |
1,2,3-Benzenetriol |
9.646 |
7.39 |
126.11 |
C6H6O3 or C6H3(OH)3 |
1057 |
antibacterial and antioxidant
|
Cynthia et al55 |
|
|
46 |
Paromomycin |
10.2 |
0.22 |
615.6 |
C23H45N5O14 |
441375 |
Antibiotic activity |
Davidson et al56 |
|
|
48 |
Tris(hydroxymethyl) nitromethane |
10.575 |
7.93 |
151.118 |
C4H9NO5 |
31337 |
Preservative, Disinfectant & Bactericidal activity
|
Izzat and Bennett57 |
|
|
49 |
Anhydro-d-mannosan |
10.839 |
0.65 |
162.14 |
C6H10O5 |
11947765 |
Muscle-relaxing and pain relieving
|
Yellu and Bhukya58 |
|
(Continued)
Nutrition and Metabolic Insights
14
|
SL. NO. |
COMPOUND |
RT |
AREA % |
MOL. WEIGHT (G/MOL) |
MOLECULAR FORMULA |
PUBCHEM CID |
CHEMICAL STRUCTURES |
BIOLOGICAL ACTIVITY |
REF |
|
50 |
2 |
10.93 |
0.34 |
322.5 |
C21H38O2 |
552098 |
Anti-microbial activity |
Srivastava et al59 |
|
|
52 |
4-Propylresorcinol |
11.842 |
1.73 |
152.19 |
C9H12O2 |
87874 |
Anti-tyrosine activity |
Matsubara et al60 |
|
|
53 |
2-(hydroxymethyl)-6- octylsulfanyloxane-3,4,5- triol |
12.04 |
0.4 |
308.44 |
C14H28O5S |
363418 |
NA |
||
|
54 |
Ethylalpha-d- glucopyranoside |
12.105 |
0.69 |
208.21 |
C8H16O6 |
91694274 |
Preservative |
Rajalakshmi and Mohan61 |
|
|
55 |
3-Deoxyhexonic acid |
12.19 |
0.25 |
180.16 |
C6H12O6 |
10350 |
NA |
||
|
56 |
Dihydroconiferyl alcohol |
12.256 |
0.39 |
182.22 |
C10H14O3 |
16822 |
Stimulating cell growth |
Lee et al62 |
|
|
57 |
2-Hydroxy-5- methylisophthalaldehyde |
14.311 |
0.28 |
164.16 |
C9H8O3 |
81744 |
NA |
||
|
58 |
(9E,11E)-octadeca-9,11- dienoate |
18.337 |
11.07 |
279.4 |
C18H31O2 |
Metabolic regulator, hypocholesterolemia, anti-atherogenic, anti- carcinogenic, antioxidant, |
Fagali and Catalá63 |
||
(Continued)
|
SL. NO. |
COMPOUND |
RT |
AREA % |
MOL. WEIGHT (G/MOL) |
MOLECULAR FORMULA |
PUBCHEM CID |
CHEMICAL STRUCTURES |
BIOLOGICAL ACTIVITY |
REF |
|
59 |
(E)-methyl octadec-11- enoate |
18.436 |
1.95 |
296.5 |
C19H36O2 |
74738 |
Anti-diarrheal activity |
||
|
60 |
13-Docosenoicacid, methyl ester (Z)- |
24.971 |
0.1 |
352.6 |
C23H44O2 |
5363109 |
Anti-cancer activity |
Paudel and Pant64 |
|
|
61 |
3,3-dihydroxypropyl palmitate |
25.108 |
2.24 |
689.1 |
C40H80O8 |
24698 |
Anti-cancer |
Zhu et al65 |
|
|
62 |
1,3-dihydroxypropan-2-yl (9Z,12Z)-octadeca-9,12- dienoate |
27.855 |
9.06 |
354.5 |
C21H38O4 |
5365676 |
Analgesic, anti-inflammatory and Anti-ulcerogenic |
Mohammed et al66 |
|
|
63 |
2,3-Dihydroxypropyl 12-hydroxyoctadecanoate |
28.288 |
0.55 |
374.6 |
C21H42O5 |
95408 |
NA |
64 9-Octadecenamide 29.195 13.7 281.5 C18H35NO 1930 O
|
hypolipidemic properties |
||||||||
|
NH2 |
||||||||
|
65 Squalene |
29.654 |
0.08 |
410.7 |
C30H50 |
638072 |
anticancer, antioxidant, drug carrier, detoxifier, skin hydrating, and emollient |
Kim and Karadeniz68 |
|
|
66 4,6-cholestadienol |
33.363 |
0.13 |
384.6 |
C27H44O |
14795191 |
Broad spectrum antimicrobial activity |
Zhu et al69 |
|
Antioxidative and
Cheng et al67
Hossain et al
15
67 Vitamin E 34.093 0.1 430.7 C29H50O2 14985 Antioxidant activity Stanner and Weichselbaum70
(Continued)
Nutrition and Metabolic Insights
16
|
SL. NO. |
COMPOUND |
RT |
AREA % |
MOL. WEIGHT (G/MOL) |
MOLECULAR FORMULA |
PUBCHEM CID |
CHEMICAL STRUCTURES |
BIOLOGICAL ACTIVITY |
REF |
|
68 |
Gamma-Tocotrienol |
34.698 |
0.13 |
410.63 |
C28H42O2 |
5282349 |
Antitumor activity |
Suman et al71 |
|
|
69 |
Ergost-5-en-3-ol, (3beta,24R)- |
35.798 |
0.31 |
400.7 |
C28H48O |
6428659 |
Antioxidant, Anti-cancerous properties |
Kang et al72 |
|
|
70 |
Alpha-tocopherol acetate |
36 |
0.18 |
472.7 |
C31H52O3 |
86472 |
antioxidant |
Tucker and Townsend73 |
|
|
71 |
Stigmasterol |
36.234 |
0.68 |
412.7 |
C29H48O |
5280794 |
Antimicrobial, anticancer, diuretic, anti-inflammatory, antioxidant |
Khalid et al74 |
|
|
72 |
Gamma sitosterol |
37.372 |
3.54 |
414.7 |
C29H50O |
457801 |
Anti-hyperglycemic |
Sirikhansaeng et al75 |
|
|
73 |
24-Propylidenecholest-5- en-3beta-ol |
37.647 |
0.29 |
426.7 |
C30H50O |
193212 |
Anti-bacterial |
Kavita et al76 |
|
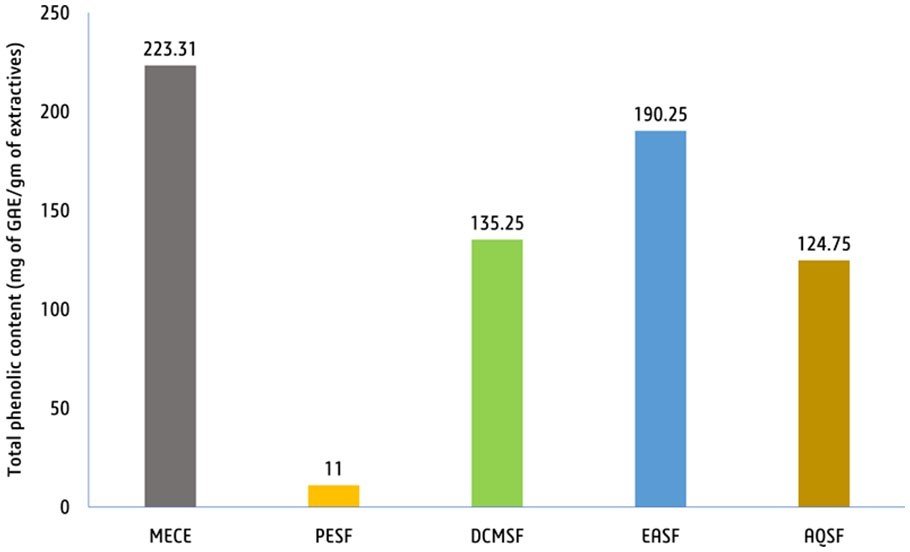
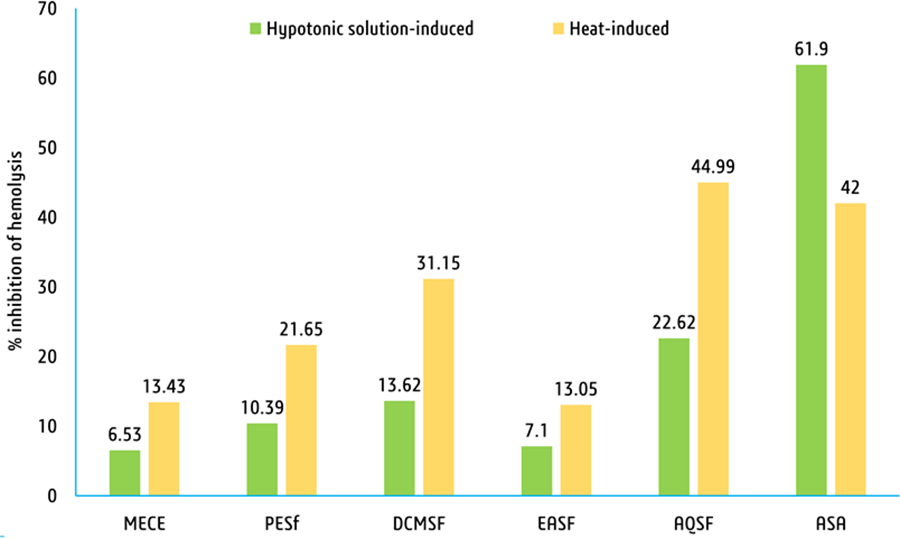
Figure 2. Total phenolic content of the various fractions and methanolic extract of V. vinifera seeds. (Here, MECE = methanolic crude extract of V. vinifera, PESF = petroleum ether soluble fraction, DCMSF = dichloromethane soluble fraction, EASF = ethyl acetate soluble fraction, and AQ = aqueous or water-soluble fraction).
Figure 4. Membrane stabilizing impact of MECE and various fractions on hypotonic medium hemolysis and heat-induced hemolysis. (Here,
MECE = methanolic crude extract of V. vinifera, PESF = petroleum ether soluble fraction, DCMSF = dichloromethane soluble fraction, EASF = ethyl acetate soluble fraction, and AQ = aqueous or water-soluble fraction).
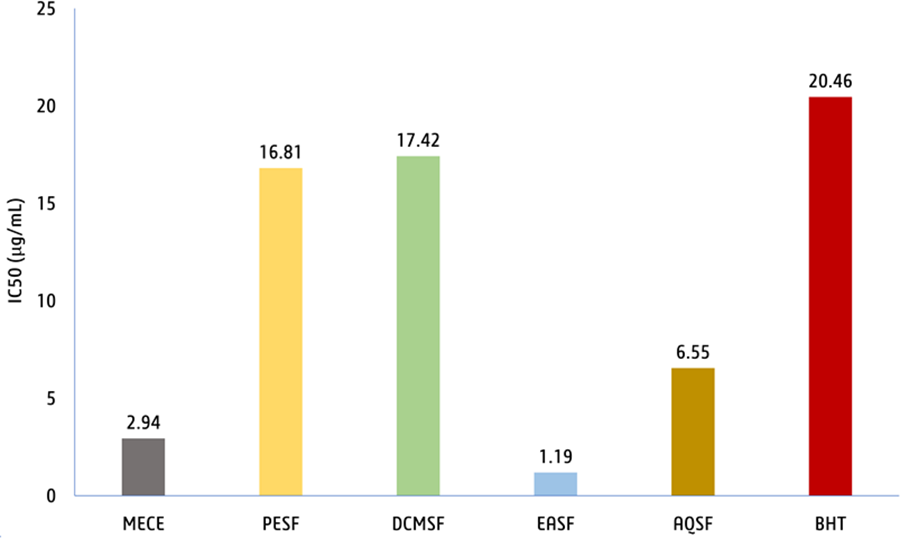
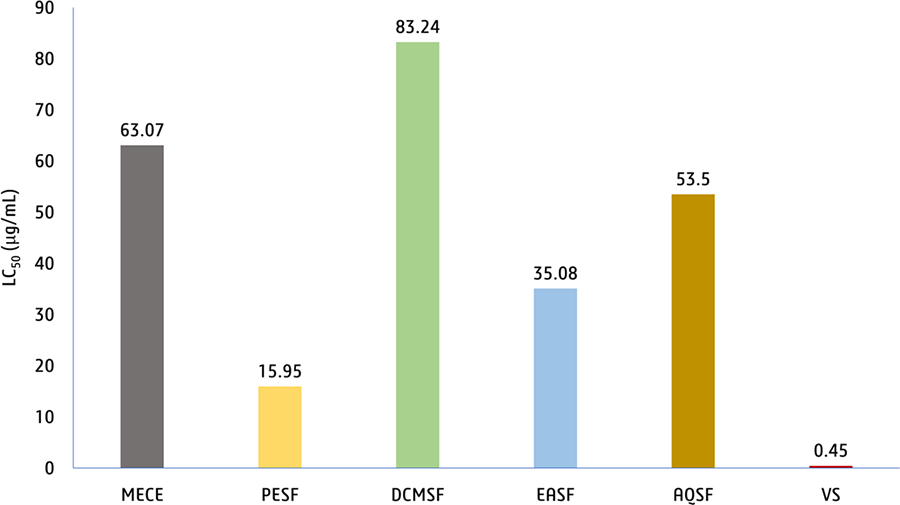
Figure 3. DPPH free radical scavenging activity (IC50, g/mL) of various solvent fractions and methanolic extract of V. vinifera seeds. (Here, MECE = methanolic crude extract of V. vinifera, PESF = petroleum ether soluble fraction, DCMSF = dichloromethane soluble fraction, EASF = ethyl acetate soluble fraction, and AQ = aqueous or water-soluble fraction).
Figure 5. Cytotoxicity of methanolic extract and its fractions of V. vinifera seeds in terms of LC50 (µg/mL). (Here, MECE = methanolic crude extract of V. vinifera, PESF = petroleum ether soluble fraction,
DCMSF = dichloromethane soluble fraction, EASF = ethyl acetate soluble fraction, and AQ = aqueous or water-soluble fraction).
acid), esters (Decyl tetradecyl ester carbonic acid), aliphatic hydrocarbons (Limonene, Nonane,5-(1-methylpropyl), Cyclopropane, Squalene), ketones (2,4-dihydroxy-2,5-dimeth- ylfuran-3(2H)-one, 2,4-dihydroxy-2,5-dimethylfuran-3(2H)- one, 1-(furan-2-yl)-2-methylprop-2-en-1-one, Maple lactone), alcohols (1,3-Propanediol,2-methyl-2-propyl, carveol), amine (Tranylcypromine), phenols (2-methoxy phenol, 1,2,4-Benzenetriol, Catechol, 4-Methyl catechol), steroids (gamma sitesterol, 24-Propylidenecholest-5-en-3beta-ol, stig- masterol, ergost-5-en-3-ol, (3beta, 24R)-), and tocopherols (alpha-tocopherol, gamma-tocotrienol; Table 1). These 73 compounds from the MECE may contribute to the medicinal activities of the fruit. From the previous literature study, most of the detected compounds in this study have anti-inflammatory, antioxidant, antibacterial, and anticancer effects (Table 1). The availability of cyclic unsaturated compound especially which has an aromatic ring such as catechol and 1,2,4-Benzenetriol may play a role for antioxidant properties of the MECE.38,44 Another molecule squalene was also identified from this plant which have potential anticancer and antioxidant activities that are matched with the grape fruits bioproperties.68
Free radicals and oxidants are harmful to the body because they are produced by both natural cellular processes and external causes such as emissions, smoking, medicines and radiation. The buildup of free radicals exceeds the body’s ability to remove them, resulting in oxidative stress.This mechanism has a crucial involve- ment in the onset of degenerative and chronic illnesses such as cardiovascular disease, autoimmune disorders, carcinoma, rheu- matoid arthritis, cataracts, aging, and neurological diseases. The human body uses a variety of strategies to resist oxidative stress, including the creation of antioxidants. These antioxidants can be produced naturally by the body or obtained from food sources or supplementation.77 In this study, the MECE showed the highest total phenolic content and EASF showed the highest scavenging capacity against DPPH freer radical. Zeghad et al78 showed that whole fruits with seeds had tremendous antioxidant activity. Another study revealed that V. vinifera is the most abundant source of various flavonoids, polyphenols, and caffeic acid deriva- tives, while V. vinifera showed positive outcomes in the DPPH and ferric reducing antioxidant power (FRAP) assays.79
Due to the harmful nature of a crude medicinal substance
which is a significant worry, a low-cost and dependable method,
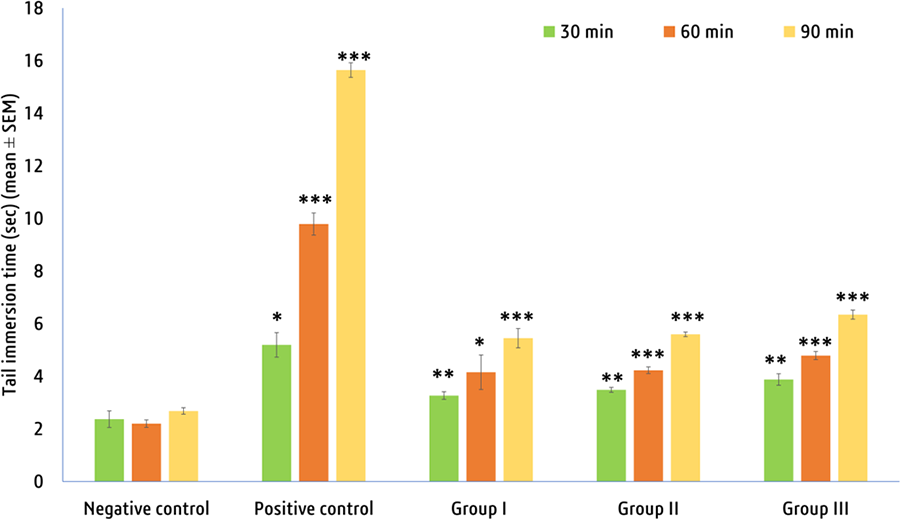
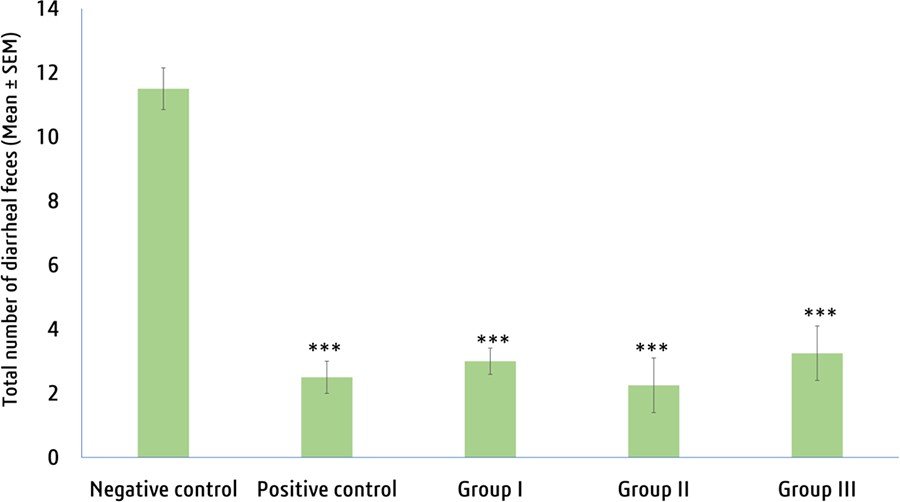
Figure 6. Central analgesic properties of crude methanolic extract of V. vinifera seeds. Values are expressed as Mean ± SEM (n = 4). ***P < .001,
*P < .05 compared to negative control group. Positive control (morphine at 2 mg/kg b.w.), Groups I, II, and III = 200, 400, and 600 mg/kg
bw = Methanol extract V. vinifera seed, respectively.
Figure 8. Effects crude methanolic extract on the number of diarrheal feces (mean ± SEM) of mice after 4h of administration of castor oil. “***” means P < .001 compared to negative control group. Positive control (loperamide at 50 mg/kg b.w.), Groups I, II, and III = 200, 400, and 600 mg/ kg bw = Methanol extract V. vinifera seeds, respectively.
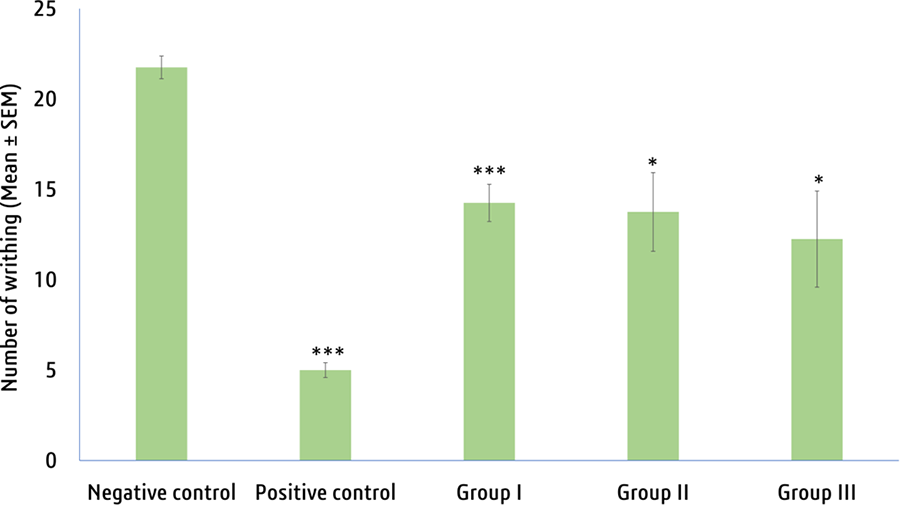
Figure 7. Peripheral analgesic properties of crude methanolic extract of
V. vinifera seeds. Values are expressed as Mean ± SEM (n = 4).
***P < .001, *P < .05 compared to negative control group. Positive control (Morphine at 2 mg/kg b.w.), Groups I, II, and III = 200, 400, and 600 mg/kg bw = Methanol extract V. vinifera seeds, respectively.
the brine shrimp lethality bioassay using Artemia salina, was per- formed to initially screen for cellular toxicity in plant extracts. A study conducted by Lagartoparra et al80 discovered a strong connection (with a correlation coefficient of .85 and a significance level of P < .05) between the 50% lethal concentration (LC50) derived from the brine shrimp test and the 50% lethal dose (LD50) from animal trials. This indicates that the brine shrimp test could serve as an alternative model. Meyer et al81 suggested that a bioactive plant compound would generally have an LC50 value of <1000 mg/mL. The present research revealed that the LC50 values obtained from the brine shrimp bioassay were all below 1000 mg/mL. As demonstrated by previous research (LC50
>10 mg/mL), none of the plant’s crude extracts or fractions should be deemed extremely toxic or deadly.82
The current research has also exerted a promising anti- inflammatory effect in heat-induced membrane stabilizing assays. Previous research has shown that V. vinifera seed extract has considerable anti-inflammatory effects by significantly reducing gene expression as well as protein secretion of inflammatory factors like tumor necrosis factor (TNF-a), interleu- kin-6 (IL-6), the inducible isoform of nitric oxide synthase (iNOS), and nitric oxide (NO).83 Through histological
examination, another study using mice found that seed extracts successfully diminished the levels of inflammatory molecules such as TNF-a , NF-K, IKK-a, IL-6, and IL-1.84 However, the findings of this current study imply that the AQSF with heat- induced hemolysis showed a similar significance anti-inflammatory effect compared to the standard drug (44.99% versus 42.00%, respectively). In the GC-MS analysis, some anti- inflammatory compounds such as 2-Methoxy 4-vinylphenol, 9,12,15-Octadecatrienoic acid and 1,3-dihydroxypropan-2-yl (9Z,12Z)-octadeca-9,12-dienoate, and stigmasterol were detected, which may responsible for the anti-inflammatory properties of the MECE. Furthermore, large antioxidant molecules detection may also be responsible for the anti-inflammatory action of the fruit. The inflammation is mainly brought on by oxidative stress.85 Histamine, serotonin, proinflammatory cytokines (including interleukin-1B and tumor necrosis factor-a), and inflammatory cells like leukotrienes and macrophages
are the mediators involved in the complicated inflammation process.85 Thromboxane A2, prostaglandins, and leukotrienes are examples of arachidonic acid metabolic products that also play a role in this process. Finally, these inflammations cause pain and diarrhea.86 As a result, the presence of antioxidant molecules in V. vinifera is advantageous since they aid in controlling inflammation by neutralizing damaging ROS. These may help prevent or treat inflammatory-related disorders and positively impact general health.
Natural remedies for pain relief are being sought as replacements to manmade medications since they are associated with side effects.87 In this study, we found that the seed extract of V. vinifera significantly decreased both central and peripheral pain experience in mice. Several analgesic compounds such as 2-meth- oxy 4-vinylphenol, Anhydro-d-mannosan, 1,3-dihydroxypropan- 2-yl (9Z,12Z)-octadeca-9,12-dienoate were also recorded from the presence study. Moreover, the predominant antioxidant molecules also conclude the potential analgesic properties of MECE. Nadia et al showed that V. vinifera has dose-dependent central analgesic activity.88 Aouey et al indicated in their study that some compounds mainly caffeoyltartaric acid and flavonoids
derivatives were detected from the V. vinifera extract that may interfere the prostaglandins pathways.89 Antioxidant molecules effectively inhibit the production of the prostaglandins and COX-2 production, which are responsible for the pain reaction.90 In addition, endogenous fatty acid amides have been shown in previous research to exhibit significant bioactivity both centrally and peripherally.87
The current study also found significant antidiarrheal effects of the MECE. Castor oil induces diarrhea primarily through 3 mechanisms: producing nitric oxide, increasing gastrointestinal membrane calcium permeability, and triggering prostaglandin production, resulting in increased fluid and electrolytes in the intestine, and stimulating peristalsis.91 The key ingredient in castor oil, ricinoleic acid, is alleged to upset the gut wall by generating prostaglandins and inducing peristaltic movement that might lead to diarrhea.92-94 Antioxidants molecules have significant prostaglandin inhibition activity.95 In this current study, although, no significant anti-diarrhea compounds were identified, several antioxidant compounds were detected from this GC-MS analysis, which may be responsible for the inhibition of castor oil-induced gut inflammation diarrhea effects.
Limitations and future research
The study presents certain areas for improvement, notably the need for comprehensive chromatographic isolation, purification, and spectroscopic characterization of the phytoconstituents from the investigated sample. Future research can be done to isolate pure compounds from the fruit’s seed extract and evaluate their pharmacological potential via in vitro, in vivo, and in silico analyses against diverse therapeutic targets.
Conclusion
The current study identified 73 phytoconstituents, including 9-octadecenamide, gamma-sitosterol, stigmasterol, paromomycin, 4,6-cholestadienol, gamma-tocotrienol, 24-Pro- pylidenecholest-5-en-3beta-ol, and alpha-tocopherol acetate, from the seed extract of V. vinifera. The study also evaluated its pharmacological properties, focusing on antioxidant, anti- inflammatory, cytotoxicity, analgesic, and antidiarrheal activities. The methanolic seed extract and its various solvent fractions exerted promising antioxidant and anti-inflammatory properties, and the 3 tested doses (200, 400, and 600 mg/kg bw) of the crude extract showed significant in vivo effects against pain and diarrhea. However, further investigation is required to completely comprehend the precise processes and to discover the bioactive substances that regulate the actions of
V. vinifera via in vitro, in vivo, and in silico approaches. Nevertheless, the antioxidant ability of this plant species pro- vides hope for reducing inflammation and enhancing health.
Acknowledgements
We would like to express our sincere appreciation to the Department of Pharmacy, State University of Bangladesh, for
their kind assistance in assisting the research by providing lab- oratory facilities.
Author Contributions
JH: Conceptualization, Writing – original draft, Writing – review & editing, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation. KRL: Visualization, Validation, Software, Resources, Methodology, Formal analysis, Data curation. AS: Writing – original draft, Visualization, Validation, Software, Resources, Methodology, Investigation,Formal analysis,Data curation.RA: Visualization, Validation, Software, Resources, Methodology, Formal analy- sis, Data curation. MAR: Writing – review & editing, Visualization, Validation, Project administration. MAA: Writing – review & editing, Visualization, Validation, Software, Resources, Data curation.
Consent for Publication
Not applicable.
Data Availability Statement
The article contains all necessary information to support the conclusions. Contacting the corresponding author with a fair request will get you further raw data.
Ethical Approval
The work adhered to the standards for the ethical treatment of animals in research set forth by the Federation of European Laboratory Animal Science Associations (FELASA). The Animal Ethics Committee of the State University of Bangladesh thoroughly reviewed and approved the research’s ethical standards and practices.
SuPPlEMENtAl MAtERIAl
Supplemental material for this article is available online.
REFERENCES
- Kumoro AC, Alhanif M, Wardhani DH. A critical review on tropical fruits seeds as prospective sources of nutritional and bioactive compounds for func- tional foods development: a case of Indonesian exotic fruits. Int J Food Sci. 2020;2020:4051475.
- Kaparapu J, Pragada PM, Geddada MN. Fruits and vegetables and its nutritional benefits. In: Egbuna C, Dable-Tupas G, eds. Functional Foods and Nutraceuticals: Bioactive Components, Formulations and Innovations. Springer, 2020;241-260.
- Allaqaband S, Dar AH, Patel U, et al. Utilization of fruit seed-based bioactive compounds for formulating the nutraceuticals and functional food: A review. Front Nutr. 2022;9:902554.
- Mitra S, Lami MS, Uddin TM, et al. Prospective multifunctional roles and pharmacological potential of dietary flavonoid narirutin. Biomed Pharmacother. 2022;150:112932.
- Mukta MM, Hossain MJ, Akter M, et al. Cardioprotection of water spinach (Ipomoea aquatica), wood apple (Limonia acidissima) and linseed (Linum usita- tissimum L.) on doxorubicin-induced cardiotoxicity and oxidative stress in rat model. Nutr Metab Insights. 2023;16:11786388231212116.
- Sarwar S, Hossain MJ, Irfan NM, et al. Renoprotection of selected antioxidant- rich foods (water spinach and red grape) and probiotics in gentamicin-induced nephrotoxicity and oxidative stress in rats. Life. 2022;12:60.
- Brennan A, Browne S. Food waste and nutrition quality in the context of public health: A scoping review. Int J Environ Res Public Health. 2021;18:5379.
- Fierascu RC, Sieniawska E, Ortan A, Fierascu I, Xiao J. Fruits By-Products – A source of valuable active principles. A short review. Front Bioeng Biotechnol. 2020;8:319.
- Hussain SZ, Naseer B, Qadri T, Fatima T, Bhat TA. Grapes (Vitis vinifera)— Morphology, taxonomy, composition and Health Benefits. Fruits Grown in High- land Regions of the Himalayas: Nutritional and Health Benefits. Hussain SZ et al., eds. Springer International Publishing; 2021;103-115.
- Radulescu C, Claudia Buruleanu L, Lucian Olteanu R, et al. Grape by-Products: potential sources of phenolic Compounds for novel functional foods. In: Bronze MR, ed. Food Science and Nutrition. IntechOpen; 2023.
- Rojas R, Castro-López C, Sánchez-Alejo EJ, Niño-Medina G, Martínez-ávila GCG. Phenolic compound recovery from grape fruit and By- products: an overview of extraction methods. In: Grape and Wine Biotechnology. InTechOpen; 2016.
- Gupta M, Dey S, Marbaniang D, et al. Grape seed extract: having a potential health benefits. J Food Sci Technol. 2020;57:1205-1215.
- Topalović A, Knežević M, Bajagić B, et al. Chapter 20 – grape (Vitis vinifera L.): health benefits and effects of growing conditions on quality parameters. In: Ozturk M, Egamberdieva D, Pešić M, eds. Biodiversity and Biomedicine. Aca- demic Press; 2020;385-401.
- VanWagenen BC, Larsen R, Cardellina JH, et al. Ulosantoin, a potent insecti- cide from the sponge Ulosa ruetzleri. J Org Chem. 1993;58:335-337.
- Harborne AJ. Phytochemical Methods a Guide to Modern Techniques of Plant Analy- sis. springer science & business media; 1998.
- Sultana C, Azad MA, Rahman MM, Muhit MA, Rahman SA. Phytochemical and biological investigation of Stevia rebaudiana (Bert.) leaves grown in Bangla- desh. Dhaka U J Pharm Sci. 2020;19:191-197.
- Hoque N, Khan ZR, Rashid PT, et al. Antimicrobial, antioxidant, and cytotoxic properties of endophytic fungi isolated from Thysanolaena maxima Roxb., Dra- caena spicata Roxb. and Aglaonema hookerianum Schott. BMC Complement Med 7er. 2023;23:347.
- Salve P, Vinchurkar A, Raut R, et al. An evaluation of antimicrobial, anticancer, anti-Inflammatory and antioxidant activities of silver nanoparticles synthesized from leaf extract of Madhuca longifolia utilizing quantitative and qualitative methods. Molecules. 2022;27(19):6404.
- Rashid PT, Hossain MJ, Zahan MS, et al. Chemico-pharmacological and com- putational studies of Ophiorrhiza fasciculata D. Don and Psychotria silhetensis Hook. f. focusing cytotoxic, thrombolytic, anti-inflammatory, antioxidant, and antibacterial properties. Heliyon. 2023;9:e20100.
- Bulbul IJ, Hossain MJ, Haque MR, et al. Two rare flavonoid glycosides from Lit- sea glutinosa (Lour.) C. B. Rob.: experimental and computational approaches endorse antidiabetic potentiality. BMC Complement Med 7er. 2024;24:69.
- Ezeja M, Omeh Y, Ezeigbo I, Ekechukwu A. Evaluation of the analgesic activity of the methanolic stem bark extract of Dialium guineense (Wild). Ann Med Health Sci Res. 2011;1:55-62.
- Rahman MM, Soma MA, Sultana N, et al. Exploring therapeutic potential of Woodfordia fruticosa (L.) Kurz leaf and bark focusing on antioxidant, antithrom- botic, antimicrobial, anti-inflammatory, analgesic, and antidiarrheal properties. Heal Sci Rep. 2023;6:e1654.
- Farzana M, Hossain MJ, El-Shehawi AM, et al. Phenolic constituents from Wendlandia tinctoria var. grandis (Roxb.) DC. Stem deciphering pharmacologi- cal potentials against oxidation, hyperglycemia, and diarrhea: phyto-pharmaco- logical and computational approaches. Molecules. 2022;27:5957.
- Goswami D, Sen S, De A. Possible antineoplastic agents, part 15: synthesis, bio- logical activity and quantitative structure activity relationship of substituted- 2-(4’-methoxybenzenesulphonamido) glutaric acid analogs against Ehrlich ascites carcinoma. Pharm. 2001;56:366-371.
- Gopal Pandit S, Honganoor Puttananjaiah M, Harohally NV, Appasaheb Dhale M. Functional attributes of a new molecule-2-hydroxymethyl-benzoic acid 2’-hydroxy-tetradecyl ester isolated from Talaromyces purpureogenus CFRM02. Food Chem. 2018;255:89-96.
- Cerny C. The aroma side of the Maillard reaction. Ann N Y Acad Sci. 2008; 1126:66-71.
- Tranylcypromine. In: LiverTox: Clinical and Research Information on Drug- Induced Liver Injury. National Institute of Diabetes and Digestive and Kidney Diseases; 2012. Accessed April 17, 2023. http://www.ncbi.nlm.nih.gov/books/ NBK548572/
- Ogata M, Matsumoto H, Kida S, et al. Synthesis and antifungal activity of a series of novel 1,2-disubstituted propenones. J Med Chem. 1987;30:1497-1502.
- Han Y, Sun Z, Chen W. Antimicrobial susceptibility and antibacterial mecha- nism of limonene against Listeria monocytogenes. Molecules. 2019;25:33.
- Kim AR, Zou Y, Kim HS, et al. Selective peroxynitrite scavenging activity of 3-methyl-1,2-cyclopentanedione from coffee extract. J Pharm Pharmacol. 2002;54:1385-1392.
- Almahboub SA, Narancic T, Devocelle M, et al. Biosynthesis of 2-aminoocta- noic acid and its use to terminally modify a lactoferricin B peptide derivative for improved antimicrobial activity. Appl Microbiol Biotechnol. 2018;102:789-799.
- Sung W, Jung H, Lee I, Kim H, Lee D. Antimicrobial Effect of Furaneol Against Human Pathogenic Bacteria and Fungi. J Microbiol Biotechnol. Published online 2006. Accessed April 17, 2023.https://scholar.google.com/scholar_looku p?title=Antimicrobial+Effect+of+Furaneol+Against+Human+Pathogenic+ Bacteria+and+Fungi&author=Sung%2C+W.S.+%28Kyungpook+National+ Un i v e r s i t y % 2 C+Da e g u% 2 C+R e p u bl ic+of+K o r e a% 29 & publ i c a t ion_year=2006
- Yale HL, Pribyl EJ, Braker W, Bernstein J, Lott WA. Muscle-relaxing com- pounds similar to 3-(o-toloxy)-1,2-propanediol. II. Substituted alkanediols. J Am Chem Soc. 1950;72:3716-3718.
- Kumar S, Koh J, Kim H, Gupta MK, Dutta PK. A new chitosan-thymine con- jugate: synthesis, characterization and biological activity. Int J Biol Macromol. 2012;50:493-502.
- Rubab M, Chelliah R, Saravanakumar K, et al. Bioactive potential of 2-methoxy- 4-vinylphenol and benzofuran from Brassica oleracea L. var. capitate f, rubra (red cabbage) on oxidative and microbiological stability of beef meat. Foods. 2020;9:568.
- Ashraf I, Zubair M, Rizwan K, et al. Chemical composition, antioxidant and antimicrobial potential of essential oils from different parts of Daphne mucro- nata Royle. Chem Cent J. 2018;12:135.
- Singh N, Mansoori A, Jiwani G, et al. Antioxidant and antimicrobial study of Schefflera vinosa leaves crude extracts against rice pathogens. Arab J Chem. 2021;14:103243.
- Zhang L, Robertson ML, Kolachana P, Davison AJ, Smith MT. Benzene metab- olite, 1,2,4-benzenetriol, induces micronuclei and oxidative DNA damage in human lymphocytes and HL60 cells. Environ Mol Mutagen. 1993;21:339-348.
- Kadiri M, Sevugapperumal N, Nallusamy S, et al. Pan-genome analysis and molecular docking unveil the biocontrol potential of Bacillus velezensis VB7 against Phytophthora infestans. Microbiol Res. 2023;268:127277.
- Junior M, Pastore G. Limonene and its oxyfunctionalized compounds: biotrans- formation by microorganisms and their role as functional bioactive compounds. Food Sci Biotechnol. 2009;18:833-841.
- Hiramoto K, Nasuhara A, Michikoshi K, Kato T, Kikugawa K. DNA strand- breaking activity and mutagenicity of 2,3-dihydro-3,5-dihydroxy-6-methyl- 4H-pyran-4-one (DDMP), a Maillard reaction product of glucose and glycine. Mutat Res Toxicol Env Mutagen. 1997;395:47-56.
- Boubakri L, Al-Ayed AS, Mansour L, et al. Bioactive NHC-derived palladium complexes: synthesis, catalytic activity for the Suzuki-Miyaura coupling of aryl chlorides and bromides and their antibacterial activities. J Coord Chem. 2019;72:2688-2704.
- Vijesh AM, Isloor AM, Shetty P, Sundershan S, Fun HK. New pyrazole deriva- tives containing 1,2,4-triazoles and benzoxazoles as potent antimicrobial and analgesic agents. Eur J Med Chem. 2013;62:410-415.
- Kocaçalışkan I, Talan I, Terzi I. Antimicrobial activity of catechol and pyrogallol as allelochemicals. Z Civilistische -forsch C. 2006;61:639-642.
- Xia YL, Cao Y, Tan XH, et al. Chemical composition and fungicidal activity of Murraya microphylla Essential oOil against Colletotrichum gloeosporioides. J Essent Oil Bearing Plants. 2020;23:678-685.
- Aberchane M, Satrani B, Fechtal M, Chaouch A. Effet de l’infection du bois de Cèdre de l’Atlas par Trametes pini et Ungulina officinalis sur la composition chi- mique et l’activité antibactérienne et antifongique des huiles essentielles. Acta Bot Gallica. 2003;150:223-229.
- Qiu Y, Lin X, Chen Z, Li B, Zhang Y. 5-Hydroxymethylfurfural exerts negative effects on gastric mucosal epithelial cells by inducing oxidative sStress, apoptosis, and tight junction disruption. J Agric Food Chem. 2022;70:3852-3861.
- Lacerda-Neto LJ, Barbosa AG, Quintans-Junior LJ, Coutinho HD, da Cunha FA. The complex pharmacology of natural products. Future Med Chem. 2019;11:797-799.
- He Q , Wang W, Zhu L. Larvicidal activity of Zanthoxylum acanthopodium essential oil against the malaria mosquitoes, Anopheles anthropophagus and Anopheles sinensis. Malar J. 2018;17:194.
- Aggarwal KK, Khanuja SPS, Ahmad A, et al. Antimicrobial activity profiles of the two enantiomers of limonene and carvone isolated from the oils of Mentha spicata and Anethum sowa. Flavour Fragrance J. 2002;17:59-63.
- Ito N, Hirose M, Imaida K. Antioxidants: Carcinogenic and Chemopreventive Properties. In: Bertino JR, ed. Encyclopedia of Cancer. 2nd ed. Academic Press; 2002;89-101.
- Ibibia E, Olabisi K, Oluwagbemiga O. Gas chromatography-mass spectrometric analysis of methanolic leaf extracts of Lannea kerstingii and Nauclea diderrichii, two medicinal plants used for the treatment of gastrointestinal tract infections. Gas. 2016;9:179-182.
- Aoki T, Uchida K. Enhanced Formation of 3-(methyithio)-1-propanol in a salt-tol- erant yeast, Zygosaccharomyces rouxii, due to deficiency of S-A denosylmethionine synthase. Agric Biol Chem. 1991;55:2113-2116.
- Yuan GF, Chen XE, Li D. Conjugated linolenic acids and their bioactivities: a review. Food Funct. 2014;5:1360-1368.
- Cynthia I, Hery S, Akhmad D. Antibacterial and antioxidant activities of pyro- gallol and synthetic pyrogallol dimer. Res J Chem Env. 2018;22:39-47.
- Davidson RN, den Boer M, Ritmeijer K. Paromomycin. Trans R Soc Trop Med Hyg. 2009;103:653-660.
- Izzat IN, Bennett EO. 7e Potentiation of the Antimicrobial Activities of Cutting Fluid Preservatives by EDTA. Univ. of Houston, TX; 1978.
- Yellu N, Bhukya B. Evaluation of anticancer activity of methanolic extract of Hiptage benghalensis (L.) Kurz on cancer cell lines. Pharmacogn Res. 2018;10:309.
- Srivastava R, Mukerjee A, Verma A. GC-MS analysis of phytocomponents in, pet ether fraction of Wrightia tinctoria seed. Pharmacogn J. 2015;7:249-253. Published online January 1, 2015. doi:10.5530/pj.2015.4.7
- Matsubara H, Kinoshita K, Koyama K, et al. Anti-tyrosinase activity of lichen metabolites and their synthetic analogues. J Hattori Bot Lab. 1997;83:179-185.
- Rajalakshmi K, Mohan V. GC-MS Analysis of Bioactive Components of Myxopyrum serratulum A.W. Hill (Oleaceae). Int J Pharm Sci. 2016;38:30-35.
- Lee TS, Purse JG, Pryce RJ, Horgan R, Wareing PF. Dihydroconiferyl alcohol
– a cell division factor from Acer species. Planta. 1981;152:571-577.
- Fagali N, Catalá A. Antioxidant activity of conjugated linoleic acid isomers, lin- oleic acid and its methyl ester determined by photoemission and DPPH tech- niques. Biophys Chem. 2008;137:56-62.
- Paudel MR, Pant B. Cytotoxic activity of crude extracts of Dendrobium amoenum and detection of bioactive compounds by GC-MS. Bot Orient J Plant Sci. 2017;11:38-42.
- Zhu S, Jiao W, Xu Y, et al. Palmitic acid inhibits prostate cancer cell prolifera- tion and metastasis by suppressing the PI3K/Akt pathway. Life Sci. 2021;286: 120046.
- Mohammed YH, Ghaidaa JM, Imad HH. Analysis of bioactive chemical com- pounds of Nigella sativa using gas chromatography-mass spectrometry. J Phar- macogn Phytother. 2016;8:8-24.
- Cheng MC, Ker YB, Yu TH, et al. Chemical synthesis of 9(Z)-octadecenamide and its hypolipidemic effect: A bioactive agent found in the essential oil of moun- tain celery seeds. J Agric Food Chem. 2010;58:1502-1508.
- Kim SK, Karadeniz F. Chapter 14 – biological importance and applications of squalene and squalane. In: Kim SK, ed. Advances in Food and Nutrition Research. Vol. 65. Marine Medicinal Foods. Academic Press; 2012;223-233.
- Zhu YZ, Liu JW, Wang X, et al. Anti-BACE1 and antimicrobial activities of steroidal compounds isolated from marine urechis unicinctus. Mar Drugs. 2018; 16:94.
- Stanner S, Weichselbaum E. Antioxidants. In: Caballero B, ed. Encyclopedia of Human Nutrition. 3rd ed. Academic Press; 2013;88-99.
- Suman S, Datta K, Chakraborty K, et al. Gamma tocotrienol, a potent radiopro- tector, preferentially upregulates expression of anti-apoptotic genes to promote intestinal cell survival. Food Chem Toxicol. 2013;60:488-496.
- Kang JH, Jang JE, Mishra SK, et al. Ergosterol peroxide from Chaga mushroom (Inonotus obliquus) exhibits anti-cancer activity by down-regulation of the - catenin pathway in colorectal cancer. J Ethnopharmacol. 2015;173:303-312.
- Tucker JM, Townsend DM. Alpha-tocopherol: roles in prevention and ther- apy of human disease. Biomed Pharmacother Bioméd Pharmacother. 2005;59: 380-387.
- Khalid A, Algarni AS, Homeida HE, et al. Phytochemical, cytotoxic, and anti- microbial evaluation of Tribulus terrestris L., Typha domingensis Pers., and Ricinus communis L.: scientific evidences for folkloric uses. Evid-Based Complement Altern Med ECAM. 2022;2022:6519712.
- Sirikhansaeng P, Tanee T, Sudmoon R, Chaveerach A. Major phytochemical as
-sitosterol disclosing and toxicity testing in Lagerstroemia species. Evid Based Complement Alternat Med. 2017;2017:7209851.
- Kavita K, Singh VK, Jha B. 24-branched 5 sterols from Laurencia papillosa red
seaweed with antibacterial activity against human pathogenic bacteria. Microbiol Res. 2014;169:301-306.
- Alam S, Dhar A, Hasan M, et al. Antidiabetic potential of commonly available fruit plants in Bangladesh: updates on prospective phytochemicals and their reported MoAs. Molecules. 2022;27:8709.
- Zeghad N, Ahmed E, Belkhiri A, Heyden YV, Demeyer K. Antioxidant activity of Vitis vinifera, Punica granatum, Citrus aurantium and Opuntia ficus indica fruits cultivated in Algeria. Heliyon. 2019;5:e01575.
- Moldovan ML, Carpa R, Fizeșan I, et al. Phytochemical profile and biological activities of tendrils and leaves extracts from a variety of Vitis vinifera L. Antioxi- dants. 2020;9:373.
- Lagartoparra A, Yhebra RS, Sardiñas IG, Buela LI. Comparative study of the assay of and the estimate of the medium lethal dose (LD50 value) in mice, to determine oral acute toxicity of plant extracts. Phytomedicine. 2001;8: 395-400.
- Meyer BN, Ferrigni NR, Putnam JE, et al. Brine shrimp: a convenient general bioassay for active plant constituents. Planta Med. 1982;45:31-34.
- Jannat T, Hossain MJ, El-Shehawi AM, et al. Chemical and pharmacological profiling of Wrightia coccinea (roxb. Ex hornem.) sims focusing antioxidant, cytotoxic, antidiarrheal, hypoglycemic, and analgesic properties. Molecules. 2022;27:4024.
- Harbeoui H, Hichami A, Wannes WA, et al. Anti-inflammatory effect of grape (Vitis vinifera L.) seed extract through the downregulation of NF-B and MAPK pathways in LPS-induced RAW264.7 macrophages. S Afr J Bot. 2019;125:1-8.
- Giribabu N, Karim K, Kilari EK, Kassim NM, Salleh N. Anti-inflammatory, antiapoptotic and proproliferative effects of Vitis vinifera seed ethanolic extract in the liver of streptozotocin-nicotinamide-induced type 2 diabetes in male rats. Can J Diabetes. 2018;42:138-149.
- Geronikaki AA, Gavalas AM. Antioxidants and inflammatory disease: syn- thetic and natural antioxidants with anti-inflammatory activity. Comb Chem High 7roughput Screen. 2006;9:425-442.
- Schirbel A, Reichert A, Roll S, et al. Impact of pain on health-related quality of life in patients with inflammatory bowel disease. World J Gastroenterol. 2010;16:3168-3177.
- Amee KNS, Hossain MJ, Rohoman A, et al. Phytochemical and pharmacologi- cal profiling of extracts of Pterygota alata (Roxb.) R. Br. leaves deciphered thera- peutic potentialities against pain, hyperglycemia and diarrhea via in vivo approaches. Pharmacol Res Prod. 2024;4:100060.
- Nadia Z, Aicha M, Sihem H, Abdelmalik B. In vivo analgesic activities and safety assessment of Vitis vinifera L and Punica granatum L fruits extracts. Trop J Pharm Res. 2017;16:553.
- Aouey B, Samet AM, Fetoui H, Simmonds MSJ, Bouaziz M. Anti-oxidant, anti-inflammatory, analgesic and antipyretic activities of grapevine leaf extract (Vitis vinifera) in mice and identification of its active constituents by LC-MS/ MS analyses. Biomed Pharmacother. 2016;84:1088-1098.
- Laube M, Kniess T, Pietzsch J. Development of antioxidant COX-2 inhibitors as radioprotective agents for radiation therapy-a hypothesis-driven review. Antioxi- dants. 2016;5:14.
- Kaur M, Singh A, Kumar B. Comparative antidiarrheal and antiulcer effect of the aqueous and ethanolic stem bark extracts of Tinospora cordifolia in rats. J Adv Pharm Technol Res. 2014;5:122-128.
- Agunu A, Yusuf S, Andrew GO, Zezi AU, Abdurahman EM. Evaluation of five medicinal plants used in diarrhoea treatment in Nigeria. J Ethnopharmacol. 2005;101:27-30.
- Hu J, Gao WY, Ling NS, Liu CX. Antidiarrhoeal and intestinal modulatory activities of Wei-chang-an-wan extract. J Ethnopharmacol. 2009;125:450-455.
- Zewdie KA, Bhoumik D, Wondafrash DZ, Tuem KB. Evaluation of in-vivo antidiarrhoeal and in-vitro antibacterial activities of the root extract of Brucea antidysenterica J. F. Mill (Simaroubaceae). BMC Complement Med 7er. 2020;20: 201-211.
- Panganamala RV, Miller JS, Gwebu ET, Sharma HM, Cornwell DG. Differen- tial inhibitory effects of vitamin E and other antioxidants on prostaglandin syn- thetase, platelet aggregation and lipoxidase. Prostaglandins. 1977;14:261-271.
Share this content: